开发酮化不饱和糖作为多功能半胱氨酸靶向共价弹头
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
多功能半胱氨酸靶向共价弹头在药物化学和化学生物学领域具有巨大的治疗潜力。在此,我们介绍了新型不饱和和不对称酮(噁唑啉烯)支架,它能在正常生理条件下选择性地与肽的半胱氨酸残基和牛血清白蛋白共轭。这种不饱和糖会消耗 NCI-H1299 细胞中的 GSH,从而在体外产生抗肿瘤作用。糖环上酮基的乙酰基可通过一次合成转化为其他羧酸。这样,负载的酸就可以在半胱氨酸共轭过程中点击释放,从而使噁唑啉苷成为一种潜在的多功能治疗剂。噁唑啉苷与 GSH 共轭的反应动力学模型已经确立,并被用于评估噁唑啉苷的反应活性。上述噁唑啉苷是通过腈与糖的一步反应立体选择性合成的,并方便地转化为一系列α,β-不饱和酮N-糖苷作为常用的合成构件。通过计算研究了恶唑啉苷合成的反应机理,并通过对照实验进行了验证。总之,这些噁唑啉苷可以很容易地合成并开发成带有有用的释放基团的半胱氨酸靶向共价弹头。多功能半胱氨酸靶向共价弹头在药物化学和化学生物学领域具有巨大的治疗潜力。在本文中,作者从腈基和糖类化合物中开发出一种噁唑啉烯支架,它可以在生理条件下选择性地轭合肽和蛋白质中的半胱氨酸残基,还可以消耗癌细胞中的谷胱甘肽。本文章由计算机程序翻译,如有差异,请以英文原文为准。
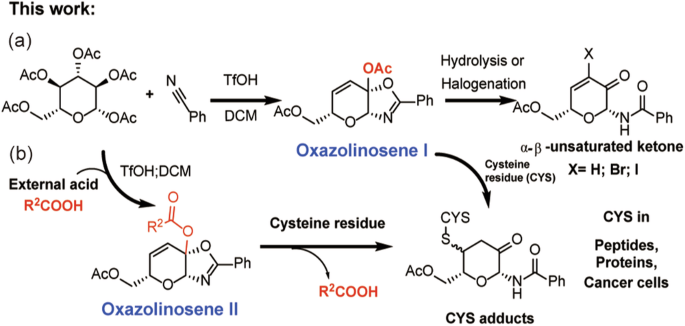
Development of ketalized unsaturated saccharides as multifunctional cysteine-targeting covalent warheads
Multi-functional cysteine-targeting covalent warheads possess significant therapeutic potential in medicinal chemistry and chemical biology. Herein, we present novel unsaturated and asymmetric ketone (oxazolinosene) scaffolds that selectively conjugate cysteine residues of peptides and bovine serum albumin under normal physiological conditions. This unsaturated saccharide depletes GSH in NCI-H1299 cells, leading to anti-tumor effects in vitro. The acetyl group of the ketal moiety on the saccharide ring can be converted to other carboxylic acids in a one-pot synthesis. In this way, the loaded acid can be click-released during cysteine conjugation, making the oxazolinosene a potential multifunctional therapeutic agent. The reaction kinetic model for oxazolinosene conjugation to GSH is well established and was used to evaluate oxazolinosene reactivity. The aforementioned oxazolinosenes were stereoselectively synthesized via a one-step reaction of nitriles with saccharides and conveniently converted into a series of α, β-unsaturated ketone N-glycosides as prevalent synthetic building blocks. The reaction mechanisms of oxazolinosene synthesis were investigated through calculations and validated with control experiments. Overall, these oxazolinosenes can be easily synthesized and developed as cysteine-targeted covalent warheads carrying useful click-releasing groups. Multifunctional cysteine targeting covalent warheads possess significant therapeutic potential in medicinal chemistry and chemical biology. Here, the authors develop an oxazolinosene scaffold from nitrile groups and saccharides that can selectively conjugate cysteine residues within peptides and proteins under physiological conditions, as well as deplete glutathione in cancer cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


