PIEZO 通道的机械传导机制和生理作用
IF 81.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
机械力是生命形成和功能发挥的基本物理要素。进化保守的 PIEZO 家族(包括哺乳动物中的 PIEZO1 和 PIEZO2)作为真正的机械激活阳离子通道的发现,改变了我们对机械力如何被感知并转化为生物活动的理解。在这篇综述中,我将讨论最近的结构-功能研究,这些研究说明了 PIEZO1 和 PIEZO2 如何采用其独特的结构设计和基于曲率的门控动力学,使它们能够作为具有高机械敏感性和选择性阳离子传导性的专用机械传导通道发挥作用。我还将讨论我们目前对 PIEZO 通道介导的生理和病理生理学作用的理解,包括 PIEZO1 对发育和功能平衡的依赖性调控,以及 PIEZO2 主导的触觉、触痛、本体感觉和内脏器官机械状态的互感等机械感觉。尽管 PIEZO 研究取得了显著进展,但本综述也强调了该领域的未决问题。本文章由计算机程序翻译,如有差异,请以英文原文为准。
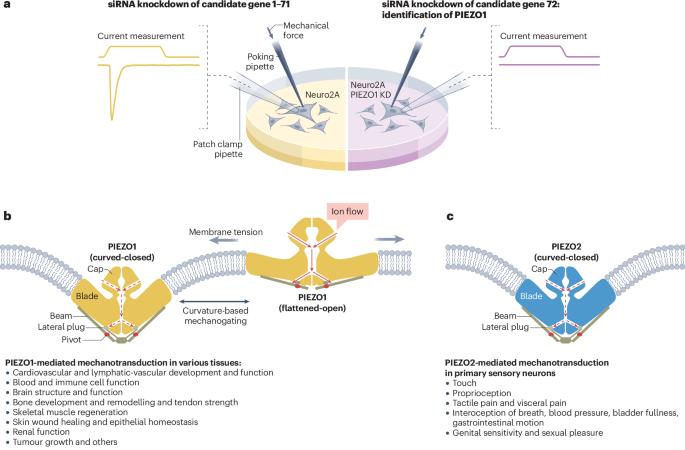
Mechanisms of mechanotransduction and physiological roles of PIEZO channels
Mechanical force is an essential physical element that contributes to the formation and function of life. The discovery of the evolutionarily conserved PIEZO family, including PIEZO1 and PIEZO2 in mammals, as bona fide mechanically activated cation channels has transformed our understanding of how mechanical forces are sensed and transduced into biological activities. In this Review, I discuss recent structure–function studies that have illustrated how PIEZO1 and PIEZO2 adopt their unique structural design and curvature-based gating dynamics, enabling their function as dedicated mechanotransduction channels with high mechanosensitivity and selective cation conductivity. I also discuss our current understanding of the physiological and pathophysiological roles mediated by PIEZO channels, including PIEZO1-dependent regulation of development and functional homeostasis and PIEZO2-dominated mechanosensation of touch, tactile pain, proprioception and interoception of mechanical states of internal organs. Despite the remarkable progress in PIEZO research, this Review also highlights outstanding questions in the field. The mechanically activated cation channels PIEZO1 and PIEZO2 are crucial for mechanotransduction processes in mammals. This Review discusses the structural design and gating dynamics of PIEZO channels that enable their high mechanosensitivity, and highlights their physiological and pathological relevance.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
173.60
自引率
0.50%
发文量
118
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews Molecular Cell Biology is a prestigious journal that aims to be the primary source of reviews and commentaries for the scientific communities it serves. The journal strives to publish articles that are authoritative, accessible, and enriched with easily understandable figures, tables, and other display items. The goal is to provide an unparalleled service to authors, referees, and readers, and the journal works diligently to maximize the usefulness and impact of each article. Nature Reviews Molecular Cell Biology publishes a variety of article types, including Reviews, Perspectives, Comments, and Research Highlights, all of which are relevant to molecular and cell biologists. The journal's broad scope ensures that the articles it publishes reach the widest possible audience.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


