光诱导铁突变和 cGAS-STING 激活的 H2S 反应性铱(III)复合物用于癌症特异性治疗
IF 6.8
1区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
触发铁变态反应是一种很有前景的抗癌治疗策略,但开发一种用于癌症特异性治疗的选择性铁变态反应诱导剂仍然是一个巨大的挑战。在此,我们精心设计了一种 H2S 响应铱(III)复合物 NA-Ir,作为铁突变诱导剂。NA-Ir可选择性地点亮富含H2S的癌细胞,主要定位于线粒体,插入线粒体DNA(mtDNA),诱导mtDNA损伤,在光照射下表现出更高的抗癌活性。机理研究表明,NA-Ir 介导的 PDT 通过 ROS 生成和 GSH 耗竭引发脂质过氧化和谷胱甘肽过氧化物酶 4 下调,从而通过多种途径导致铁变态反应。此外,mtDNA 的强烈损伤可激活环 GMP-AMP 合成酶-干扰素基因刺激器(cGAS-STING)通路,导致铁蛋白吞噬和进一步的铁变态反应。RNA序列分析表明,NA-Ir介导的PDT主要影响与铁蛋白吞噬、自噬和癌症免疫相关的基因表达。这项研究首次展示了铁突变和 cGAS-STING 激活的癌症特异性实例,为多模式协同治疗提供了新策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
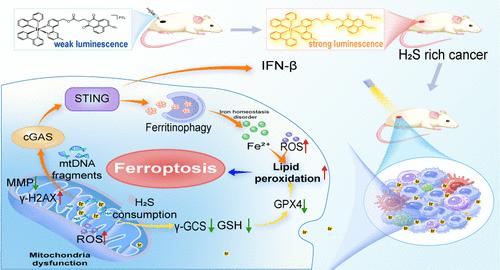
Photoinduction of Ferroptosis and cGAS-STING Activation by a H2S-Responsive Iridium(III) Complex for Cancer-Specific Therapy
Triggering ferroptosis represents a promising anticancer therapeutic strategy, but the development of a selective ferroptosis inducer for cancer-specific therapy remains a great challenge. Herein, a H2S-responsive iridium(III) complex NA-Ir has been well-designed as a ferroptosis inducer. NA-Ir could selectively light up H2S-rich cancer cells, primarily localize in mitochondria, intercalate into mitochondrial DNA (mtDNA), and induce mtDNA damage, exhibiting higher anticancer activity under light irradiation. Mechanistic studies showed that NA-Ir-mediated PDT triggered lipid peroxidation and glutathione peroxidase 4 downregulation through ROS production and GSH depletion, resulting in ferroptosis through multiple pathways. Moreover, the intense mtDNA damage can activate the cyclic GMP–AMP synthase-stimulator of the interferon gene (cGAS-STING) pathway, leading to ferritinophagy and further ferroptosis. RNA-sequencing analysis showed that NA-Ir-mediated PDT mainly affects the expression of genes related to ferroptosis, autophagy, and cancer immunity. This study demonstrates the first cancer-specific example with ferroptosis and cGAS-STING activation, which provides a new strategy for multimodal synergistic therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Medicinal Chemistry
医学-医药化学
CiteScore
4.00
自引率
11.00%
发文量
804
审稿时长
1.9 months
期刊介绍:
The Journal of Medicinal Chemistry is a prestigious biweekly peer-reviewed publication that focuses on the multifaceted field of medicinal chemistry. Since its inception in 1959 as the Journal of Medicinal and Pharmaceutical Chemistry, it has evolved to become a cornerstone in the dissemination of research findings related to the design, synthesis, and development of therapeutic agents.
The Journal of Medicinal Chemistry is recognized for its significant impact in the scientific community, as evidenced by its 2022 impact factor of 7.3. This metric reflects the journal's influence and the importance of its content in shaping the future of drug discovery and development. The journal serves as a vital resource for chemists, pharmacologists, and other researchers interested in the molecular mechanisms of drug action and the optimization of therapeutic compounds.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


