磺酰胺的对映选择性 Chan-Lam S- 芳基化反应
IF 44.6
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
含硫立体分子对药物开发具有重要影响。其中,亚磺酰亚胺是具有 S(IV)立体中心的手性分子,在化学和生物学中具有重要价值,但迄今为止,实现这种分子的合成仍具有挑战性。同样,控制 Chan-Lam 偶联中的立体化学也是一项挑战,这种偶联已被广泛用于通过使用铜络合物将亲核物与硼酸偶联来构建 C-N、C-O 和 C-S 键。在此,我们报告了亚磺酰胺与芳基硼酸的高度化学选择性和对映体选择性 Chan-Lam S-芳基化反应,从而得到一系列热力学上不被看好的含有硫立体中心的芳基亚磺酰亚胺。我们设计了一种由 2-吡啶基 N-苯基二氢咪唑配体组成的铜催化剂,通过明确的手性环境和高反应活性实现了有效的对映体控制,从而超越了背景外消旋转化。实验与计算相结合的研究确定了反应机理,并揭示了化学选择性和立体选择性的来源。本文章由计算机程序翻译,如有差异,请以英文原文为准。
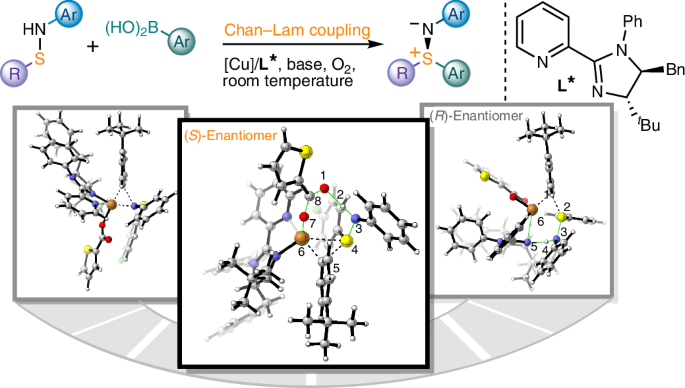
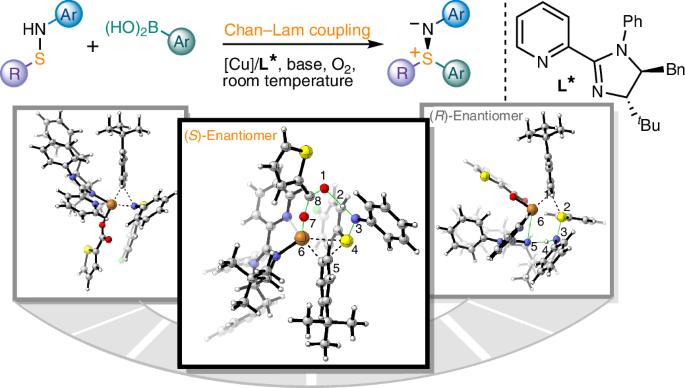
Enantioselective Chan–Lam S-arylation of sulfenamides
Sulfur stereogenic molecules have a significant impact on drug development. Among them, sulfilimines are chiral molecules bearing S(IV) stereocentres, which exhibit great value in chemistry and biology but have so far been synthetically challenging to achieve. Similarly, it has also been a challenge to control the stereochemistry in Chan–Lam coupling, which has been widely used to construct C–N, C–O and C–S bonds by coupling nucleophiles with boronic acids using copper complexes. Here we report a highly chemoselective and enantioselective Chan–Lam S-arylation of sulfenamides with arylboronic acids to deliver an array of thermodynamically disfavoured aryl sulfilimines containing a sulfur stereocentre. A copper catalyst from a 2-pyridyl N-phenyl dihydroimidazole ligand has been designed that enables effective enantiocontrol by means of a well-defined chiral environment and high reactivity that outcompetes the background racemic transformation. A combined experimental and computational study establishes the reaction mechanism and unveils the origin of chemoselectivity and stereoselectivity. Sulfilimines are a class of chiral molecules that bear S(IV) stereocentres, which are of high value in drug discovery but difficult to synthesize. Now the authors report a chemo- and enantioselective Chan–Lam S-arylation of sulfenamides with arylboronic acids that delivers diaryl and alkyl aryl sulfilimines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Catalysis
Chemical Engineering-Bioengineering
CiteScore
52.10
自引率
1.10%
发文量
140
期刊介绍:
Nature Catalysis serves as a platform for researchers across chemistry and related fields, focusing on homogeneous catalysis, heterogeneous catalysis, and biocatalysts, encompassing both fundamental and applied studies. With a particular emphasis on advancing sustainable industries and processes, the journal provides comprehensive coverage of catalysis research, appealing to scientists, engineers, and researchers in academia and industry.
Maintaining the high standards of the Nature brand, Nature Catalysis boasts a dedicated team of professional editors, rigorous peer-review processes, and swift publication times, ensuring editorial independence and quality. The journal publishes work spanning heterogeneous catalysis, homogeneous catalysis, and biocatalysis, covering areas such as catalytic synthesis, mechanisms, characterization, computational studies, nanoparticle catalysis, electrocatalysis, photocatalysis, environmental catalysis, asymmetric catalysis, and various forms of organocatalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


