NSD2 是促进前列腺肿瘤发生的 AR/FOXA1 新增强体的必要亚基
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
雄激素受体(AR)是一种配体反应型转录因子,可驱动前列腺管腔上皮细胞的终末分化。相比之下,在源自这些细胞的肿瘤中,AR染色质占位被广泛重编程以激活恶性表型,其分子机制仍不清楚。在这里,我们发现肿瘤特异性AR增强子主要依赖于NSD2的H3K36二甲基转移酶活性。前列腺癌异常诱导 NSD2 的表达,NSD2 的失活破坏了 65% 以上的前列腺癌细胞色素体,从而损害了 AR 的转录潜能。NSD2依赖的AR位点明显含有嵌合的FOXA1:AR半位点,这些位点完全包括从患者标本中定义的肿瘤特异性AR增强子回路。NSD2 失活还会增加对 NSD1 旁系亲属的依赖性,而双 NSD1/2 PROTAC 降解剂在 AR 依赖性前列腺癌模型中具有优先细胞毒性。总之,我们将 NSD2 定性为一种重要的 AR 新增强子亚基,这种亚基使其具有致癌活性,并将 NSD1/2 定位为晚期前列腺癌的可行共同靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
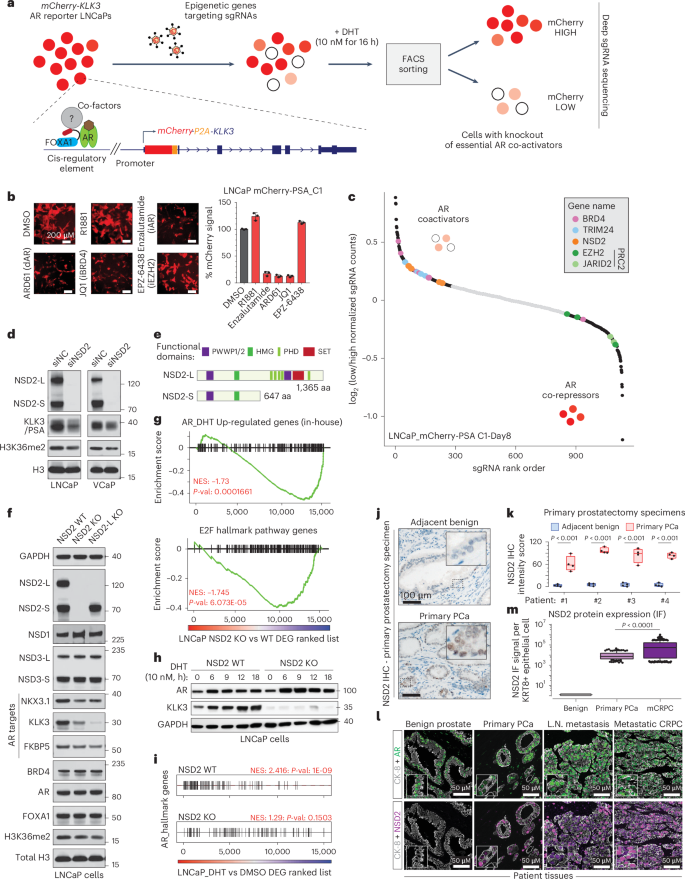
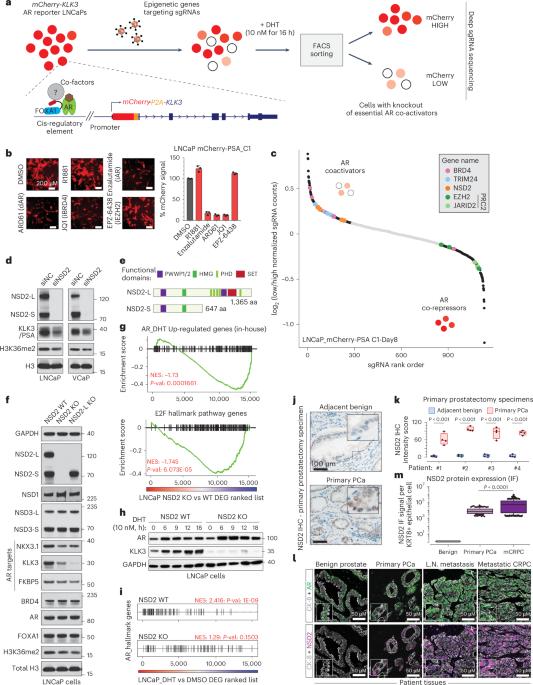
NSD2 is a requisite subunit of the AR/FOXA1 neo-enhanceosome in promoting prostate tumorigenesis
Androgen receptor (AR) is a ligand-responsive transcription factor that drives terminal differentiation of the prostatic luminal epithelia. By contrast, in tumors originating from these cells, AR chromatin occupancy is extensively reprogrammed to activate malignant phenotypes, the molecular mechanisms of which remain unknown. Here, we show that tumor-specific AR enhancers are critically reliant on H3K36 dimethyltransferase activity of NSD2. NSD2 expression is abnormally induced in prostate cancer, where its inactivation impairs AR transactivation potential by disrupting over 65% of its cistrome. NSD2-dependent AR sites distinctively harbor the chimeric FOXA1:AR half-motif, which exclusively comprise tumor-specific AR enhancer circuitries defined from patient specimens. NSD2 inactivation also engenders increased dependency on the NSD1 paralog, and a dual NSD1/2 PROTAC degrader is preferentially cytotoxic in AR-dependent prostate cancer models. Altogether, we characterize NSD2 as an essential AR neo-enhanceosome subunit that enables its oncogenic activity, and position NSD1/2 as viable co-targets in advanced prostate cancer. CRISPR screen identifies coactivators of the androgen receptor (AR) complex, including NSD2. NSD2 contributes to AR cistrome reprogramming during prostate cancer progression, and its degradation via a novel PROTAC reduces prostate cancer cell viability in vitro.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


