2′,3′-环磷酸 tRNA 修复功能受损导致水稻热敏基因雄性不育
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
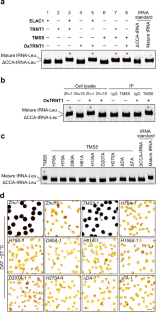
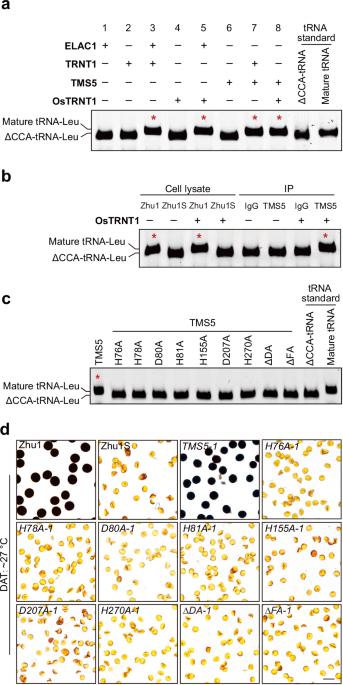
杂交水稻在亚洲广泛种植,具有抗病原性和优异的产量,是全球粮食安全的主要贡献者。双系杂交水稻系统利用表现出光敏/热敏基因雄性不育(P/TGMS)的突变体,是最主要的杂交水稻育种技术。在目前的双系杂交水稻育种技术中,95%以上的双系杂交水稻品系都存在对热敏感基因雄性不育5(TMS5)的突变。我们以前曾发现 TMS5 带有核糖核酸酶 ZS1 的突变。尽管 TMS5 对培育健壮的水稻品系非常重要,但其介导的 TGMS 机理仍然难以捉摸。在这里,我们证明了 TMS5 是一种 tRNA 2′,3′-环磷酸酶。tms5 突变会导致 2′,3′-环磷酸(cP)-ΔCCA-tRNAs(以 cP 结尾的不含 3′ CCA 的 tRNAs)的积累,高温会加剧这种积累,并降低成熟 tRNAs 的丰度,尤其是丙氨酸 tRNAs(tRNA-Alas)。在 tms5 突变体中过表达 tRNA-Alas 可使雄性繁殖力恢复到 70%。值得注意的是,通过敲除编码 cP-ΔCCA-tRNA 生成酶的 OsVms1,tms5 突变体的雄性繁殖力在高温下完全恢复。我们的研究揭示了水稻中 tms5 介导的 TGMS 的基本机制,为进一步改进杂交作物发育中的 TGMS 提供了机制上的启示。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Impaired 2′,3′-cyclic phosphate tRNA repair causes thermo-sensitive genic male sterility in rice
Hybrid rice, widely planted in Asia, is pathogen resistant and has superior yields, making it a major contributor to global food security. The two-line hybrid rice system, which utilizes mutants exhibiting photo-/thermo-sensitive genic male sterility (P/TGMS), is the leading hybrid rice breeding technology. Mutations in THERMO-SENSITIVE GENIC MALE STERILE 5 (TMS5) accounts for over 95% of current TGMS lines. We previously found that tms5 carries a mutation in ribonuclease ZS1. Despite its importance for breeding robust rice lines, the mechanism underlying tms5-mediated TGMS remains elusive. Here, we demonstrate that TMS5 is a tRNA 2′,3′-cyclic phosphatase. The tms5 mutation leads to accumulation of 2′,3′-cyclic phosphate (cP)-ΔCCA-tRNAs (tRNAs without 3′ CCA ended with cP), which is exacerbated by high temperatures, and reduction in the abundance of mature tRNAs, particularly alanine tRNAs (tRNA-Alas). Overexpression of tRNA-Alas in the tms5 mutant restores male fertility to 70%. Remarkably, male fertility of tms5 mutant is completely restored at high temperatures by knocking out OsVms1 which encodes the enzyme for cP-ΔCCA-tRNA generation. Our study reveals the mechanism underlying tms5-mediated TGMS in rice and provides mechanistic insight into the further improvement of TGMS in hybrid crop development.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


