人类尿酸盐转运体 URAT1 的转运机制和结构药理学。
IF 28.1
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
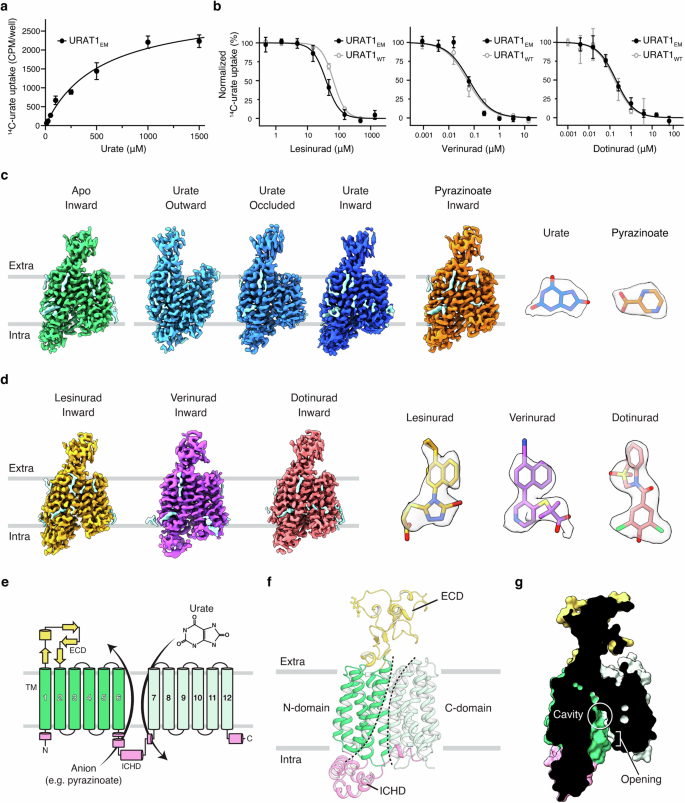
尿酸盐是肝脏中嘌呤代谢的内源性产物。血液中尿酸盐含量过高会导致痛风,痛风是一种非常常见且痛苦的炎症性关节炎。排出的尿酸盐主要通过URAT1反转运体在肾脏重吸收,URAT1反转运体是抗痛风药物的一个关键靶点。为了揭示尿酸盐转运和药物抑制的机制,我们测定了人URAT1与尿酸盐、反阴离子吡嗪酸盐或不同化学类型的抗痛风药物(lesinurad、verinurad和dotinurad)的冷冻电镜结构。我们捕捉到了URAT1在尿酸盐吸收过程中从外向内的转变,揭示了尿酸盐与一个富含苯丙氨酸的口袋结合,并与关键的门控残基结合以驱动转运循环。与尿酸盐的单一结合位点不同,吡嗪酸盐与URAT1内三个不同的功能相关位点相互作用,这种机制尚未在其他阴离子拮抗剂中观察到。此外,我们还发现,虽然所有这三种药物都会与底物竞争并停止转运循环,但verinurad 和 dotinurad 会进一步劫持门控残基以获得高效力。这些见解增进了我们对有机阴离子转运的了解,为设计更好的痛风治疗药物奠定了基础。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Transport mechanism and structural pharmacology of human urate transporter URAT1
Urate is an endogenous product of purine metabolism in the liver. High urate levels in the blood lead to gout, a very common and painful inflammatory arthritis. Excreted urate is reabsorbed in the kidney mainly by URAT1 antiporter, a key target for anti-gout drugs. To uncover the mechanisms of urate transport and drug inhibition, we determined cryo-EM structures of human URAT1 with urate, counter anion pyrazinoate, or anti-gout drugs of different chemotypes — lesinurad, verinurad, and dotinurad. We captured the outward-to-inward transition of URAT1 during urate uptake, revealing that urate binds in a phenylalanine-rich pocket and engages with key gating residues to drive the transport cycle. In contrast to the single binding site for urate, pyrazinoate interacts with three distinct, functionally relevant sites within URAT1, a mechanism that has not yet been observed in other anion antiporters. In addition, we found that while all three drugs compete with substrates and halt the transport cycle, verinurad and dotinurad further hijack gating residues to achieve high potency. These insights advance our understanding of organic anion transport and provide a foundation for designing improved gout therapeutics.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Cell Research
生物-细胞生物学
CiteScore
53.90
自引率
0.70%
发文量
2420
审稿时长
2.3 months
期刊介绍:
Cell Research (CR) is an international journal published by Springer Nature in partnership with the Center for Excellence in Molecular Cell Science, Chinese Academy of Sciences (CAS). It focuses on publishing original research articles and reviews in various areas of life sciences, particularly those related to molecular and cell biology. The journal covers a broad range of topics including cell growth, differentiation, and apoptosis; signal transduction; stem cell biology and development; chromatin, epigenetics, and transcription; RNA biology; structural and molecular biology; cancer biology and metabolism; immunity and molecular pathogenesis; molecular and cellular neuroscience; plant molecular and cell biology; and omics, system biology, and synthetic biology. CR is recognized as China's best international journal in life sciences and is part of Springer Nature's prestigious family of Molecular Cell Biology journals.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


