调查化学多样性:邻丙炔基苯酚是 2-取代苯并呋喃和苯并二氢吡喃分歧合成中的关键化合物。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
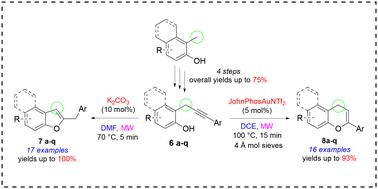
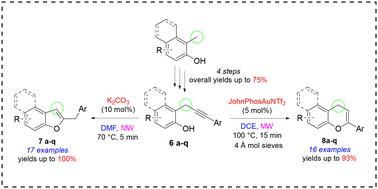
在本研究中,我们从 17 种取代的邻丙炔基苯酚(其特征是丙炔sp3 碳上的单芳基取代)出发,探索并优化了一种用于合成 2-取代苯并呋喃和苯并色烯的 MW 增强发散方法。首先,我们开发了一个用于制备邻丙炔基苯酚库的强大平台。在碱性条件下,邻丙炔基苯酚发生区域选择性反应生成苯并呋喃,产率从 43% 到 100% 不等。相反,在阳离子金催化条件下,我们能够获得相应的 4H-色烯,尽管产率变化更大(从 25% 到 93%),而且区域选择性略低。我们还提出了解释所观察到的不同结果的合理机制。我们的发现强调了以多样性为导向的合成在研究分子复杂性方面的潜力。事实证明,我们忽视的邻丙炔基苯酚是通过分子内环化反应获得含氧杂环支架的多功能战略起始材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Investigating chemical diversity: o-propargylphenols as key compounds in the divergent synthesis of 2-substituted benzofurans and chromenes†
In this study, we explored and optimized a MW-enhanced divergent approach for the synthesis of 2-substituted benzofurans and chromenes, starting from seventeen substituted o-propargylphenols characterized by a monoaryl substitution on the propargylic sp3 carbon. Firstly, we developed a robust platform for the preparation of a library of o-propargylphenols. Under basic conditions, o-propargylphenols reacted regioselectively to yield benzofurans in yields ranging from 43% to 100%. Conversely, under cationic gold catalysis, we were able to obtain the corresponding 4H-chromenes, albeit in more variable yields (from 25% to 93%) and slightly lower regioselectively. We also proposed plausible mechanisms to explain the divergent outcomes observed. Our findings underscore the potential of diversity-oriented synthesis in the investigation of molecular complexity. Our neglected o-propargylphenols have proven to be versatile and strategic starting materials for accessing oxygen-containing heterocyclic scaffolds through intramolecular cyclization reactions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


