利用对 MMP-2 敏感的 nHA/GNE 共包水凝胶调节免疫抑制微环境,加强黑色素瘤治疗。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
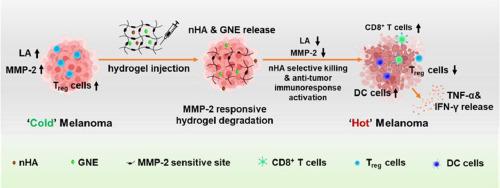
乳酸和基质金属蛋白酶(MMPs)过表达等免疫抑制性肿瘤微环境已被证实对肿瘤治疗不利。本研究利用具有选择性抗肿瘤作用的纳米羟基磷灰石(nHA)、乳酸脱氢酶 A 抑制剂 (R)-GNE-140(GNE)和基质金属蛋白酶-2(MMP-2)敏感肽的协同作用,成功研制出一种治疗黑色素瘤的肿瘤微环境调控水凝胶。这种水凝胶是由 4-arm-聚乙二醇-马来酸酐(4-arm-PEG-MAL)和 MMP-2 敏感肽(CC-14)反应生成的,其中 nHA 和 GNE 通过物理方式共同包裹在水凝胶中。体外降解试验证实,与中性或不含 MMP-2 的条件相比,在酸性较低(pH 值为 6.8)且含有 MMP-2 的条件下,nHA 和 GNE 可从水凝胶中加速释放,这表明制备的水凝胶具有 pH 值和 MMP-2 响应特性。体外细胞实验结果表明,水凝胶可以通过乳酸代谢失调使细胞周期堆积,从而阻止黑色素瘤细胞的增殖,并通过 nHA 的直接杀伤作用促进细胞凋亡。此外,经过水凝胶处理后,黑色素瘤细胞的迁移率和侵袭性都明显降低。一项体内抗黑色素瘤研究表明,水凝胶能明显抑制肿瘤生长,使CD8+T细胞和抗原递呈细胞增多,而Treg细胞浸润减少,最终提高了疗效。因此,制备的水凝胶在治疗黑色素瘤方面大有可为,可以成为一种高效治疗黑色素瘤的新策略。意义说明:迄今为止,生物材料纳米羟基磷灰石(nHA)已被证明具有选择性杀死癌细胞的能力。该研究报告了一种肿瘤微环境(TME)调节水凝胶,目的是通过将 nHA 给药与免疫抑制微环境调节相结合来提高黑色素瘤的疗效。制备的水凝胶对 pH 和 MMP-2 敏感。因此,可以观察到 nHA 和乳酸脱氢酶 A 抑制剂(GNE)的可控释放,并在肿瘤部位发生原位 MMP-2 消耗。此外,水凝胶还能通过诱导乳酸代谢失调,叠加细胞周期,以及利用 nHA 直接杀死细胞,促进细胞凋亡,从而有效抑制黑色素瘤细胞的生长。此外,水凝胶还能增加 CD8+ T 细胞和抗原递呈细胞的生成,同时减少 Treg 细胞在肿瘤部位的浸润。这可以将最初的 "冷 "肿瘤转化为 "热 "肿瘤,最终提高治疗效果。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Enhancing melanoma therapy by modulating the immunosuppressive microenvironment with an MMP-2 sensitive and nHA/GNE co-encapsulated hydrogel
The immunosuppressive tumor microenvironment, such as lactic acid and matrix metalloproteinases (MMPs) overexpression, has been well confirmed to be adverse for tumor therapy. In current study, a tumor microenvironment modulatory hydrogel was successfully developed to treat melanoma by taking advantage of the synergistic effects of nano-hydroxyapatite (nHA) with well-documented selective anti-tumor action, lactate dehydrogenase A inhibitor (R)-GNE-140 (GNE), and matrix metalloproteinase-2 (MMP-2) sensitive peptide. The hydrogel was acquired by the reaction of 4-arm-polyethylene glycol-maleic anhydride (4-arm-PEG-MAL) and MMP-2 sensitive peptide (CC-14), in which nHA and GNE were co-encapsulated physically. The in vitro degradation tests confirmed the accelerated release of nHA and GNE from the hydrogel under less-acidic (pH 6.8) and MMP-2 containing conditions compared to those neutral or without MMP-2 conditions, demonstrating the pH and MMP-2 responsive properties of as-prepared hydrogel. Findings from in vitro cell experiments revealed that the hydrogel could stop the proliferation of melanoma cells by stacking cell cycle via lactic acid metabolic dysregulation and boosting cell apoptosis via nHA direct killing effect. Moreover, after hydrogel treatment, the rate of migration and aggressiveness of melanoma cells both reduced significantly. An in vivo anti-melanoma study showed that the hydrogel could inhibit tumor growth significantly and result in more CD8+ T cells and antigen-presenting cells but less Treg cells infiltration, ultimately leading to an enhanced therapeutic efficacy. As thus, the fabricated hydrogel demonstrated great promise for treating melanoma and could be a new potent strategy for efficient melanoma therapy.
Statement of significance
Nano-hydroxyapatite (nHA) has the capability of selectively killing cancer cells. The study reported a tumor microenvironment (TME) modulatory hydrogel with the goal of enhancing melanoma therapy efficacy by combining nHA administration with immunosuppressive microenvironment modulation. The hydrogel demonstrated pH and MMP-2 sensitivity. Hence, controlled release of nHA and lactate dehydrogenase A inhibitor (GNE) could be observed, and in situ MMP-2 consumption at the tumor site occurred. The hydrogel effectively inhibited the growth of melanoma cells. Furthermore, hydrogel increased the production of CD8+ T cells and antigen-presenting cells while decreasing the infiltration of Treg cells at the tumor site. This could transform the initial “cold” tumor into a “hot” tumor, ultimately resulting in an enhanced therapeutic effect.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


