在耐碳青霉烯类肺炎克雷伯氏菌肺部感染过程中,肺部神经感受器感觉神经元促进了肺炎性败血症的发生。
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
耐碳青霉烯类肺炎克雷伯氏菌(CRKP)可导致革兰氏阴性肺部感染和致命的肺炎败血症,但目前的治疗方法有限。肺部有密集的痛觉神经元,它们介导呼吸、咳嗽和支气管收缩。痛觉神经元在防御革兰氏阴性肺部病原体中的作用尚不清楚。在这里,我们发现肺神经感受器能促进 CRKP 肺炎和肺炎败血症。消融小鼠的神经感受器可增加肺部CRKP的清除率,抑制CRKP的跨肺泡扩散,并保护小鼠免于低体温和死亡。此外,痛觉感受器的消融增强了中性粒细胞和 Ly6Chi 单核细胞的募集以及细胞因子的诱导。Ly6Chi单核细胞(而非嗜中性粒细胞)的耗竭会降低消融小鼠肺部和肺外CRKP的清除率,这表明Ly6Chi单核细胞是调节肺炎败血症的关键细胞群。此外,神经肽降钙素基因相关肽抑制了 Ly6Chi 单核细胞中活性氧的诱导及其杀伤 CRKP 的能力。以神经肽信号为靶点可能是治疗耐多药革兰氏阴性菌感染和肺炎败血症的一种治疗方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
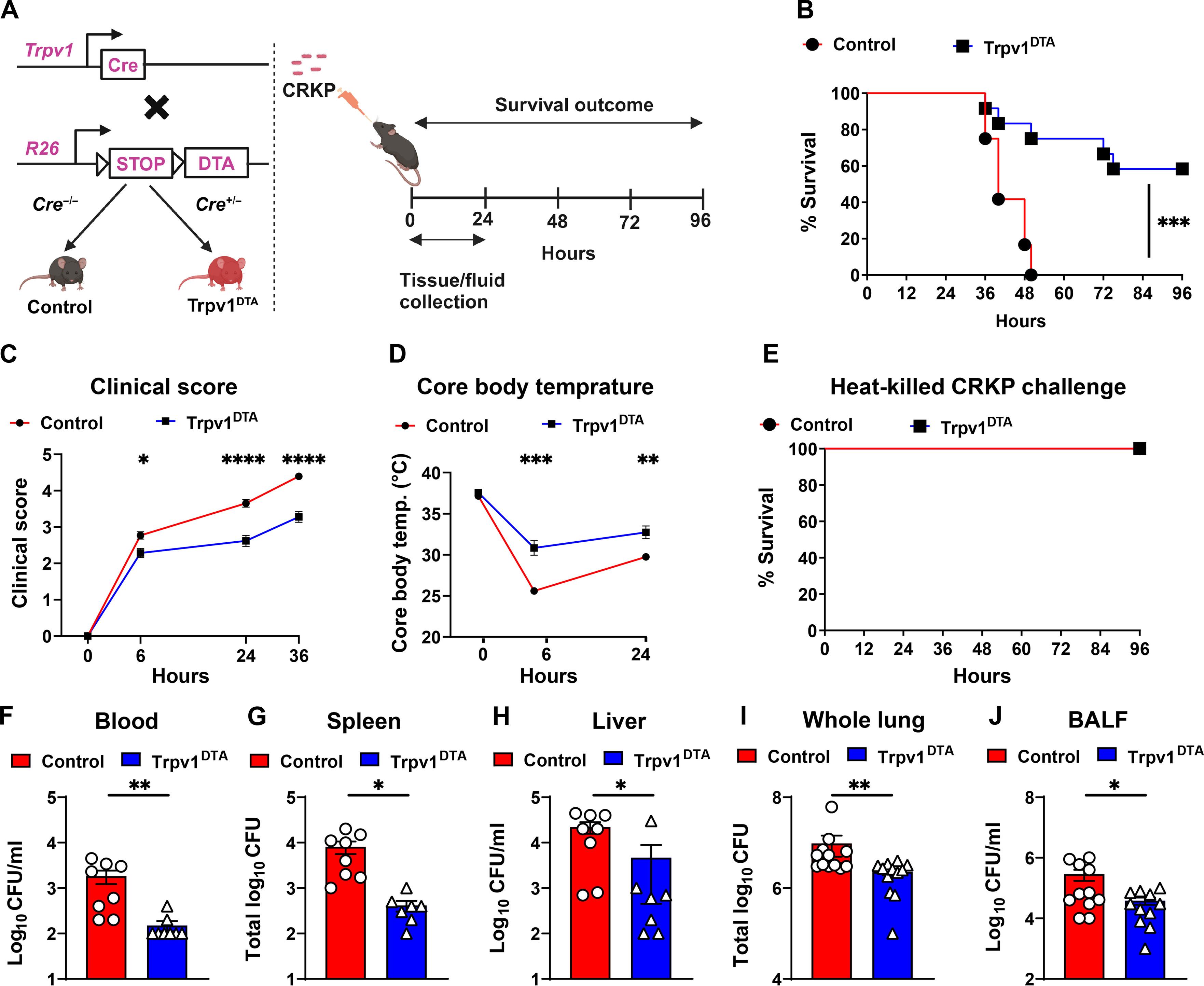
Lung-innervating nociceptor sensory neurons promote pneumonic sepsis during carbapenem-resistant Klebsiella pneumoniae lung infection
Carbapenem-resistant Klebsiella pneumoniae (CRKP) causes Gram-negative lung infections and fatal pneumonic sepsis for which limited therapeutic options are available. The lungs are densely innervated by nociceptor sensory neurons that mediate breathing, cough, and bronchoconstriction. The role of nociceptors in defense against Gram-negative lung pathogens is unknown. Here, we found that lung-innervating nociceptors promote CRKP pneumonia and pneumonic sepsis. Ablation of nociceptors in mice increased lung CRKP clearance, suppressed trans-alveolar dissemination of CRKP, and protected mice from hypothermia and death. Furthermore, ablation of nociceptors enhanced the recruitment of neutrophils and Ly6Chi monocytes and cytokine induction. Depletion of Ly6Chi monocytes, but not of neutrophils, abrogated lung and extrapulmonary CRKP clearance in ablated mice, suggesting that Ly6Chi monocytes are a critical cellular population to regulate pneumonic sepsis. Further, neuropeptide calcitonin gene–related peptide suppressed the induction of reactive oxygen species in Ly6Chi monocytes and their CRKP-killing abilities. Targeting nociceptor signaling could be a therapeutic approach for treating multidrug-resistant Gram-negative infection and pneumonic sepsis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


