镓簇促进的 In2O3 催化剂用于二氧化碳加氢制甲醇
IF 13.1
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在这项研究中,在 In2O3 上构建了高度分散的镓簇(称为 Ga/In2O3),以促进二氧化碳加氢转化为甲醇。与原始 In2O3 相比,Ga/In2O3 催化剂明显提高了 CO2 转化率和甲醇时空产率(STY),并增强了在 60 小时气流中的稳定性。多种技术证实,负载的镓和 In2O3 载体之间存在强烈的电子相互作用,从而在 In2O3 上形成了分散良好、稳定的镓簇,这有利于催化剂的长期性能。镓团簇与有缺陷的 In2O3 支持物之间的界面是促进 H2 和 CO2 活化的活性位点。这种界面协同作用不仅能增强 H2 的吸附和解离,还能有效抑制 CO2 以碳酸盐形式的强吸附。界面位点上的活化 CO2 很容易解离成吸附的 CO,从而进一步氢化成甲醇。本文章由计算机程序翻译,如有差异,请以英文原文为准。
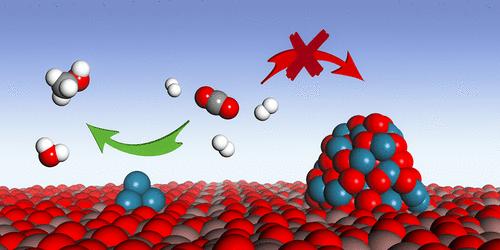
Gallium Cluster-Promoted In2O3 Catalyst for CO2 Hydrogenation to Methanol
In this work, highly dispersed gallium clusters are constructed on In2O3 (denoted as Ga/In2O3) to promote hydrogenation of CO2 to methanol. Compared to pristine In2O3, the Ga/In2O3 catalysts show markedly improved CO2 conversion and methanol space-time yield (STY), as well as enhanced stability over 60 h on stream. A strong electronic interaction between the loaded gallium and In2O3 support, as confirmed by multiple techniques, results in well-dispersed, stable gallium clusters on In2O3, which is beneficial to long-term performance. The interface between gallium clusters and the defective In2O3 support serves as active sites to facilitate the activation of H2 and CO2. This interfacial synergy not only enhances the adsorption and dissociation of H2 but also effectively inhibits the strong adsorption of CO2 as carbonate. The activated CO2 at the interface sites can be dissociated readily into adsorbed CO for further hydrogenation into methanol.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


