结核分枝杆菌毒脂 PDIM 可抑制小鼠的自噬作用
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
摘要
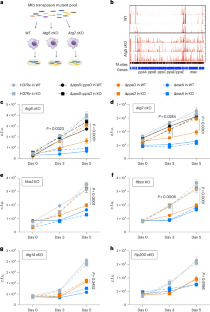
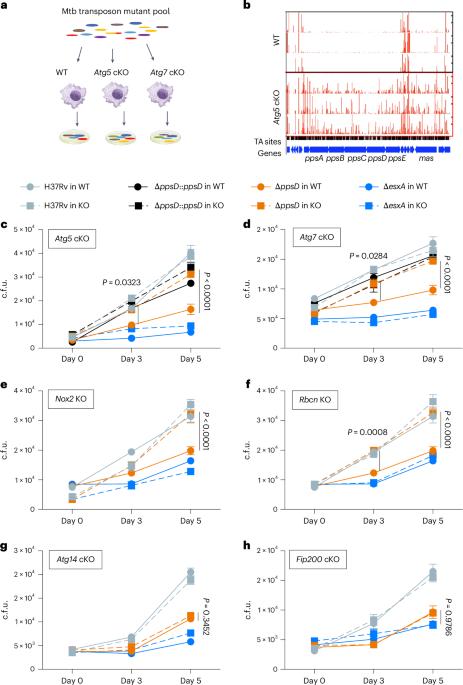
结核分枝杆菌(Mtb)会感染多种肺部巨噬细胞群,这些巨噬细胞群具有不同的限制 Mtb 的能力。Mtb在某些巨噬细胞群中存活的原因尚不清楚。在这里,我们利用转座子测序分析了野生型和缺乏ATG5或ATG7的自噬缺陷型小鼠巨噬细胞中的Mtb,发现参与噬菌醇二甲基椰油酸盐(PDIM)毒脂合成的Mtb基因具有抗自噬能力。利用ppsD突变体Mtb,我们发现PDIM通过抑制吞噬体招募NADPH氧化酶来抑制LC3相关吞噬作用(LAP)。在小鼠体内,PDIM能保护Mtb免受LAP和经典自噬的影响。在急性感染期间,PDIM对于Mtb在肺泡巨噬细胞中的存活是不可或缺的,但对于非肺泡巨噬细胞中的存活则是必需的,其方式依赖于自噬。在慢性感染期间,自噬缺陷小鼠在感染PDIM缺陷的Mtb后死亡,淋巴滤泡中的B细胞积累出现障碍。这些研究结果表明,PDIM有助于Mtb的毒力和免疫逃避,揭示了自噬在B细胞反应中的作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Mycobacterium tuberculosis virulence lipid PDIM inhibits autophagy in mice
Mycobacterium tuberculosis (Mtb) infects several lung macrophage populations, which have distinct abilities to restrict Mtb. What enables Mtb survival in certain macrophage populations is not well understood. Here we used transposon sequencing analysis of Mtb in wild-type and autophagy-deficient mouse macrophages lacking ATG5 or ATG7, and found that Mtb genes involved in phthiocerol dimycocerosate (PDIM) virulence lipid synthesis confer resistance to autophagy. Using ppsD mutant Mtb, we found that PDIM inhibits LC3-associated phagocytosis (LAP) by inhibiting phagosome recruitment of NADPH oxidase. In mice, PDIM protected Mtb from LAP and classical autophagy. During acute infection, PDIM was dispensable for Mtb survival in alveolar macrophages but required for survival in non-alveolar macrophages in an autophagy-dependent manner. During chronic infection, autophagy-deficient mice succumbed to infection with PDIM-deficient Mtb, with impairments in B-cell accumulation in lymphoid follicles. These findings demonstrate that PDIM contributes to Mtb virulence and immune evasion, revealing a contributory role for autophagy in B-cell responses. Mycobacterial phthiocerol dimycocerosate (PDIM) inhibits LC3-associated phagocytosis and counters autophagy-dependent restriction of Mycobacterium tuberculosis in non-alveolar macrophages.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


