1-芳基-1,3-丁二烯与二硫代氨基甲酸的 Regioselective Markovnikov Hydrothiolation,生成烯丙基二硫代氨基甲酸烯丙酯
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究人员开发了一种无金属、配体和溶剂的三组分反应,通过 1-芳基-1,3-丁二烯、CS2 和胺来生成烯丙基二硫代氨基甲酸烯丙酯。在这一过程中,CS2 和仲胺被用于 C-N 和 C-S 键,从而生成烯丙基二硫代氨基甲酸烯丙酯。该方法具有显著的马尔科夫尼科夫选择性,反应条件温和,操作简单,与各种官能团兼容,通过 37 个具有优异区域选择性的实例证明了这一点。这是首例二硫代氨基甲酸与不对称 1,3 二烯体系的加成反应。这也证明了它在药物分子后期官能化和放大反应方面的多功能性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
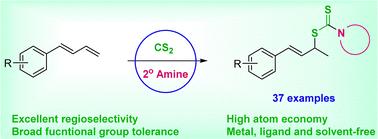
Regioselective Markovnikov hydrothiolation of 1-aryl-1,3-butadienes with dithiocarbamic acid to allyl dithiocarbamates†
A metal, ligand and solvent-free three component reaction of 1-aryl-1,3-butadienes, CS2 and amine has been developed. In this process, readily available CS2 and secondary amines were used for C-N and C-S bonds giving allyl dithiocarbamates with notable Markovnikov selectivity, mild reaction conditions, simple operation and compatibility with various functional groups (37 examples). This is first case of dithiocarbamic acid addition to an unsymmetrical 1,3-diene system.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


