人类 T 细胞白血病病毒 1 型未成熟 Gag 网格的独特稳定性
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
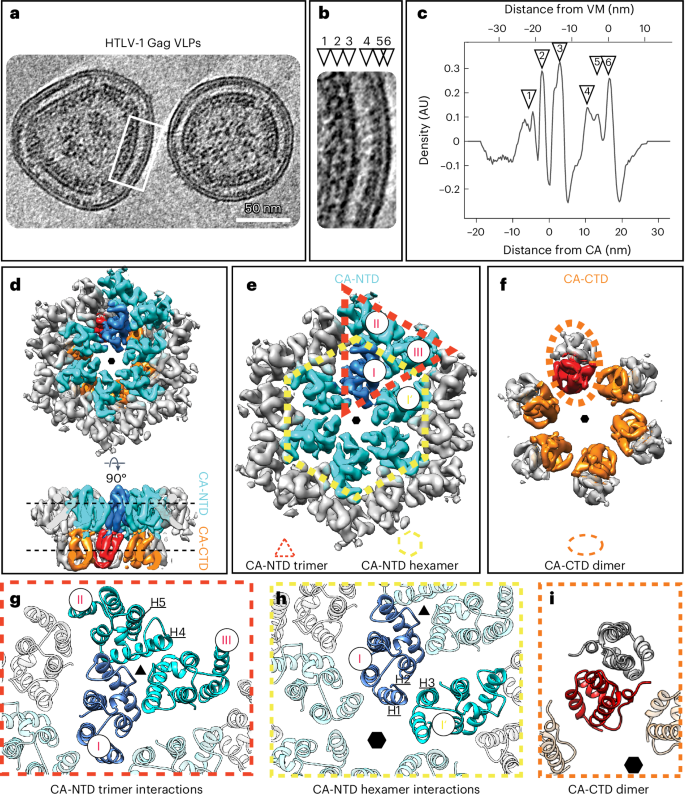
人类 T 细胞白血病病毒 1 型(HTLV-1)未成熟颗粒在形态上与其他逆转录病毒不同,表明其组装方式与众不同。在此,我们报告了对体外组装的 HTLV-1 病毒样颗粒以及来自细胞的 HTLV-1 病毒样颗粒的低温电子断层扫描研究结果。这项工作表明,HTLV-1 采用了一种独特的 Gag-Gag 相互作用机制来形成未成熟的病毒晶格。对未成熟囊膜(CA)管状阵列的高分辨率结构信息分析表明,HTLV-1 的主要稳定成分是 CA 的 N 端结构域。突变分析也支持这一观点。这将 HTLV-1 与其他逆转录病毒区分开来,后者的稳定作用主要由 CA 的 C 端结构域提供。这些结果提供了未成熟三角逆转录病毒 Gag 四元排列的结构细节,这有助于解释 HTLV-1 颗粒在形态上与众不同的原因。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Distinct stabilization of the human T cell leukemia virus type 1 immature Gag lattice
Human T cell leukemia virus type 1 (HTLV-1) immature particles differ in morphology from other retroviruses, suggesting a distinct way of assembly. Here we report the results of cryo-electron tomography studies of HTLV-1 virus-like particles assembled in vitro, as well as derived from cells. This work shows that HTLV-1 uses a distinct mechanism of Gag–Gag interactions to form the immature viral lattice. Analysis of high-resolution structural information from immature capsid (CA) tubular arrays reveals that the primary stabilizing component in HTLV-1 is the N-terminal domain of CA. Mutagenesis analysis supports this observation. This distinguishes HTLV-1 from other retroviruses, in which the stabilization is provided primarily by the C-terminal domain of CA. These results provide structural details of the quaternary arrangement of Gag for an immature deltaretrovirus and this helps explain why HTLV-1 particles are morphologically distinct. Using cryo-electron tomography of human T cell leukemia virus type 1 virus-like particles, the authors reveal that the immature Gag lattice is stabilized by the capsid N-terminal domain in contrast to other retroviruses that use the C-terminal domain.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


