Ag(I)-catalyzed diastereoselective oxidative cyclopropanation of prochiral alkyne-tethered 1,3-dicarbonitriles.
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
在此,我们以高氯酸为有效氧化剂,开发了一种高非对映选择性银催化分子内环丙烷化亲手性烷基拴1,3-二碳腈的方法。这种方法有助于高产率地构建具有三个全碳季立体中心的高功能化复合物 [6.6.5.3] 框架。通过克级反应和产物的合成后修饰,证明了该反应的重要性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
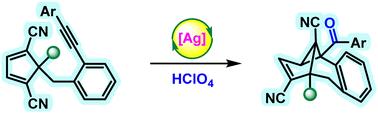
Ag(i)-catalyzed diastereoselective oxidative cyclopropanation of prochiral alkyne-tethered 1,3-dicarbonitriles†
Herein, we developed a highly diastereoselective silver-catalyzed intramolecular cyclopropanation of prochiral alkyne-tethered 1,3-dicarbonitriles using perchloric acid as an effective oxidizing agent. This method facilitates the construction of densely functionalized complex [6.6.5.3] frameworks having three all-carbon quaternary stereocenters in high yields. The significance of the reaction was demonstrated by a gram-scale reaction and post-synthetic modifications of the product.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


