利用可见光从芳香族重氮化合物合成二氢金牛酮。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-06
DOI:10.1021/acs.joc.4c01064
引用次数: 0
摘要
二氢aurones 是醛酮的衍生物,具有类似的生物活性。虽然二氢贝壳杉酮的合成方法很多,但避免使用贵金属的生态友好型方法仍有待探索。在这项工作中,通过芳香重氮化合物的环化,开发了一种无催化剂、可见光驱动的二氢乌头酮合成方法。反应在温和的条件下顺利进行,以中等到较高的产率得到了一系列二氢aurones。机理研究表明,这一过程涉及自由基对史蒂文斯重排。本文章由计算机程序翻译,如有差异,请以英文原文为准。
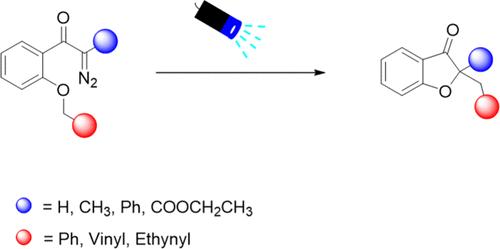
Visible-Light-Driven Synthesis of Dihydroaurones from Aromatic Diazo Compounds.
Dihydroaurones, which are derivatives of aurones, exhibit similar biological activity. Although there are many synthetic methods for dihydroaurones, ecofriendly methodologies that circumvent the use of precious metals still need to be explored. In this work, a catalyst-free, visible-light-driven synthesis of dihydroaurones has been developed through the cyclization of aromatic diazo compounds. The reaction proceeded smoothly under mild conditions, resulting in a series of dihydroaurones in moderate to high yields. Mechanistic investigation suggests that this process involves a radical-pair Stevens rearrangement.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


