核团传入神经对威胁辨别的性别差异编码促使奖赏行为受到抑制
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
学习预测威胁是至关重要的,但同样重要但却经常被忽视的是学习不存在威胁。在这里,我们通过记录厌恶性线索和中性线索时两个伏隔核(NAc)谷氨酸能传入的神经活动,揭示了威胁线索辨别的性别编码。在雄性小鼠中,来自腹侧海马的 NAc 传入神经优先被威胁线索激活。在雌性小鼠中,这些腹侧海马-NAc投射会被威胁和非威胁线索同时激活,而来自内侧前额叶皮层的NAc传入则会被脚震更强烈地调用,并可靠地区分威胁和非威胁。尽管雌雄小鼠的突触连接相似,但化学遗传通路特异性抑制发现,在线索介导的奖赏动机行为抑制中,腹侧海马-NAc和内侧前额叶皮层-NAc投射之间存在双重分离。我们认为,这些性别偏差可能反映了行为策略的性别差异,这可能与理解精神疾病风险的性别差异有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sex-biased neural encoding of threat discrimination in nucleus accumbens afferents drives suppression of reward behavior
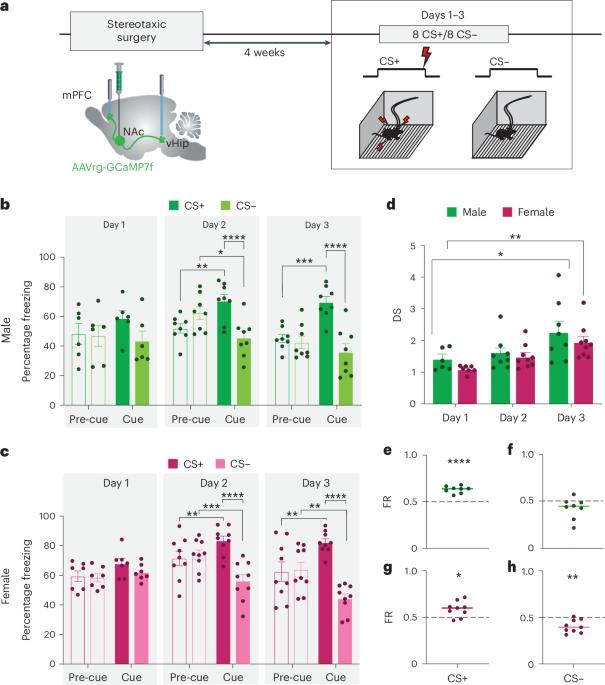
Learning to predict threat is essential, but equally important—yet often overlooked—is learning about the absence of threat. Here, by recording neural activity in two nucleus accumbens (NAc) glutamatergic afferents during aversive and neutral cues, we reveal sex-biased encoding of threat cue discrimination. In male mice, NAc afferents from the ventral hippocampus are preferentially activated by threat cues. In female mice, these ventral hippocampus–NAc projections are activated by both threat and nonthreat cues, whereas NAc afferents from medial prefrontal cortex are more strongly recruited by footshock and reliably discriminate threat from nonthreat. Chemogenetic pathway-specific inhibition identifies a double dissociation between ventral hippocampus–NAc and medial prefrontal cortex–NAc projections in cue-mediated suppression of reward-motivated behavior in male and female mice, despite similar synaptic connectivity. We suggest that these sex biases may reflect sex differences in behavioral strategies that may have relevance for understanding sex differences in risk of psychiatric disorders. Muir et al. explore threat discrimination in male and female mice and find that, despite similar behavioral acquisition, there are surprising sex differences in the neural encoding that drives suppression of reward seeking under threat.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


