光学纯(3R,4R)-1-苄基和(3R,4R)-1-叔丁氧羰基-3-甲基-4-氨基哌啶的简便、立体选择性和可扩展合成方法
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
从市场上可买到的 N-苄基-3-甲基-4-哌啶酮开始,一种高效、可扩展和立体选择性的光学纯 (3R,4R)-1-Benzyl- 和 (3R,4R)-1-Boc-3-methyl-4-aminopiperidines 合成方法应运而生。合成的关键步骤包括 N-苄基-3-甲基-4-哌啶酮的手性解析、酮基的顺式选择性还原,以及随后对所得羟基进行 Mitsunobu 反转,以安装具有所需反式立体化学结构的胺。本文所述的方法展示了一条以经济有效和可扩展的方式制备标题化合物的有效途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
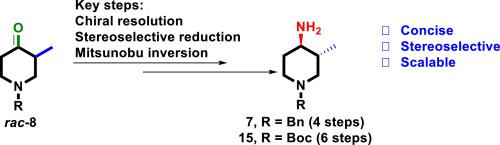
A concise, stereoselective and scalable synthesis of optically pure (3R,4R)-1-benzyl- and (3R,4R)-1-Boc-3-methyl-4-aminopiperidines
An efficient, scalable and stereoselective synthesis of optically pure (3R,4R)-1-benzyl- and (3R,4R)-1-Boc-3-methyl-4-aminopiperidines has been developed starting from commercially available N-benzyl-3-methyl-4-piperidone. The synthesis employed chiral resolution of N-benzyl-3-methyl-4-piperidone, cis-selective reduction of a keto group, and subsequent Mitsunobu inversion of the resulting hydroxy group to install an amine with the desired trans-stereochemistry as the key steps. The method described herein demonstrated a competent route to the title compounds in a cost-effective, and scalable manner.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


