基于免疫脂质体的 CRISPR/Cas9gRNA-IL30 复合物靶向递送可抑制前列腺癌并延长生存期。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
开发针对前列腺癌(PC)的选择性无毒免疫疗法具有挑战性。白细胞介素(IL)30在前列腺癌中起着免疫抑制和致癌的作用,其对肿瘤的特异性抑制可能具有重要的临床意义。利用抗PSCA抗体驱动的脂质纳米复合物(Cas9gRNA-hIL30-PSCA NxPs)在PC异种移植物中介导的CRISPR/Cas9 IL30基因缺失显示了显著的基因组编辑效率和循环稳定性,且无脱靶效应或器官毒性。每两周给PC小鼠静脉注射一次Cas9gRNA-hIL30-PSCA NxPs可抑制肿瘤的生长和转移,提高生存率。从机理上讲,Cas9gRNA-hIL30-PSCA NxPs抑制了人PC细胞中ANGPTL 1/2/4、IL1β、CCL2、CXCL1/6、SERPINE1-F1、EFNB2、PLG、PF4、VEGFA、VEGFD、ANG、TGFβ1、EGF和HGF的表达,同时上调了CDH1、DKK3和PTEN的表达,导致细胞低增殖和大面积缺血坏死。在同种异体 PC 模型中,IL30 靶向免疫脂质体下调了 NFKB1 的表达,阻止了 CD11b+Gr-1+MDCs、Foxp3+Tregs 和 NKp46+RORγt+ILC3 的瘤内流入,并通过抑制肿瘤进展延长了宿主生存期。这项研究证明,基于免疫脂质体的Cas9gRNA-IL30靶向递送是一种潜在的安全有效的PC治疗策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
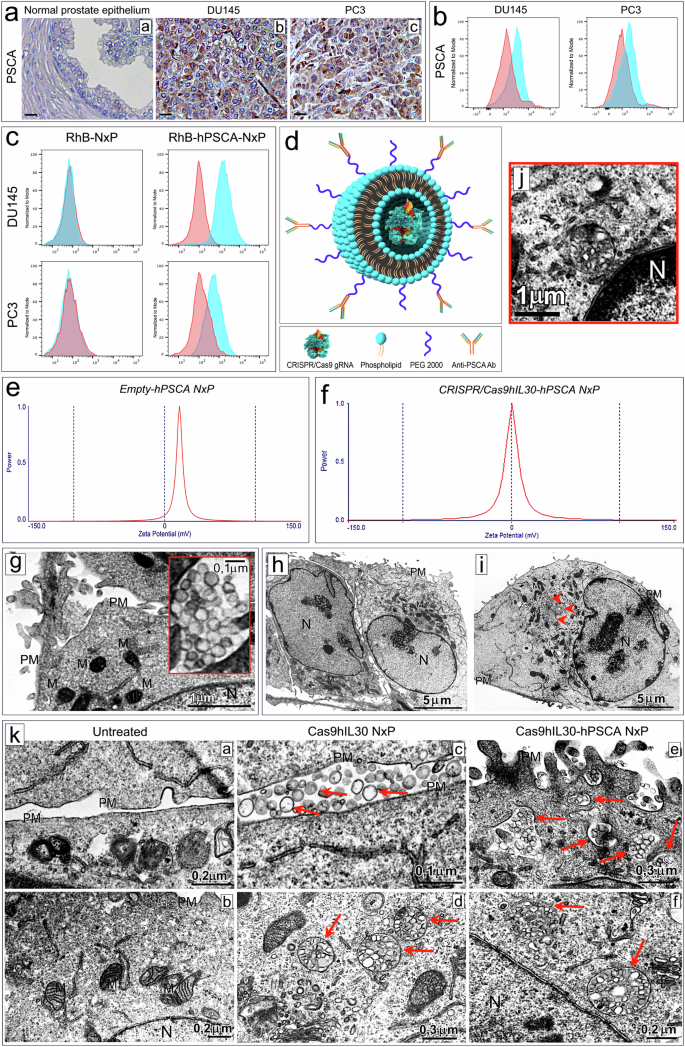
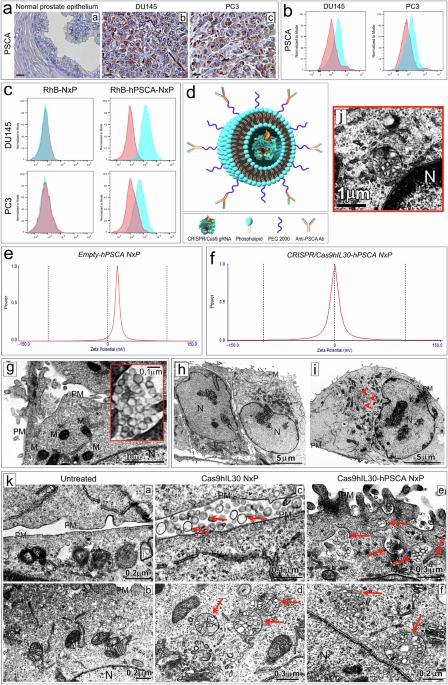
Immunoliposome-based targeted delivery of the CRISPR/Cas9gRNA-IL30 complex inhibits prostate cancer and prolongs survival
The development of selective and nontoxic immunotherapy targeting prostate cancer (PC) is challenging. Interleukin (IL)30 plays immunoinhibitory and oncogenic roles in PC, and its tumor-specific suppression may have significant clinical implications. CRISPR/Cas9-mediated IL30 gene deletion in PC xenografts using anti-PSCA antibody-driven lipid nanocomplexes (Cas9gRNA-hIL30-PSCA NxPs) revealed significant genome editing efficiency and circulation stability without off-target effects or organ toxicity. Biweekly intravenous administration of Cas9gRNA-hIL30-PSCA NxPs to PC-bearing mice inhibited tumor growth and metastasis and improved survival. Mechanistically, Cas9gRNA-hIL30-PSCA NxPs suppressed ANGPTL 1/2/4, IL1β, CCL2, CXCL1/6, SERPINE1-F1, EFNB2, PLG, PF4, VEGFA, VEGFD, ANG, TGFβ1, EGF and HGF expression in human PC cells while upregulated CDH1, DKK3 and PTEN expression, leading to low proliferation and extensive ischemic necrosis. In the syngeneic PC model, IL30-targeting immunoliposomes downregulated NFKB1 expression and prevented intratumoral influx of CD11b+Gr-1+MDCs, Foxp3+Tregs, and NKp46+RORγt+ILC3, and prolonged host survival by inhibiting tumor progression. This study serves as a proof of principle that immunoliposome-based targeted delivery of Cas9gRNA-IL30 represent a potentially safe and effective strategy for PC treatment. Prostate cancer is an age-related disease and a leading cause of cancer death in men worldwide. Although its incidence has remained stable over the past decade, the proportion of tumors diagnosed in the advanced stages is rising, highlighting the need for improved and personalized treatments, especially for older patients with additional health problems. Di Carlo and coworkers created a NanoLiposome delivery system to transport a gene-editing tool, CRISPR/Cas9, directly to prostate cancer cells, to target and inhibit a particular gene involved in cancer growth. This study tested this approach’s effectiveness and safety on human prostate cancer cells and mouse models. The findings show the potential of using NanoLiposomes for targeted gene editing in cancer. The researchers conclude that this method could provide a new and effective way to treat prostate cancer, particularly in patients requiring gentler treatment options. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


