作为甲基和过氘代甲基转移剂的环状亚磺酰亚胺及其在光氧化催化中的应用。
IF 16.9
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
苯并[1,3,2]二噻唑-1,1,3-三氧化物是一种稳定易用的试剂。在光氧化催化过程中,它们会产生甲基和过氘甲基自由基,这些自由基可与多种自由基受体相加,包括烯烃、丙烯酰胺、喹喔啉酮、异氰酸酯、烯醇硅烷和 N-Ts 丙烯酰胺。作为副产品,生成的盐可以再生为原始甲基化剂。流动化学为扩大反应规模提供了一种选择,进一步突出了这些甲基化试剂的合成用途。机理研究表明,光氧化催化诱导了单电子转移(SET)途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
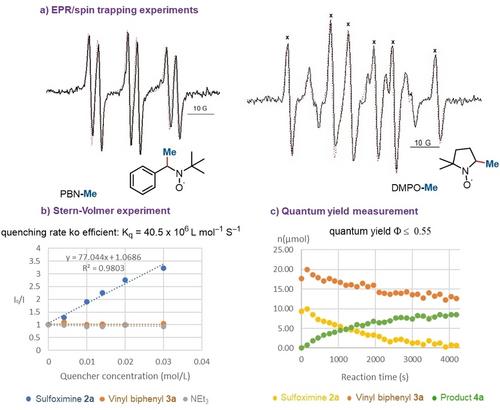
Cyclic Sulfoximines as Methyl and Perdeuteromethyl Transfer Agents and Their Applications in Photoredox Catalysis
Benzo[1,3,2]dithiazole-1,1,3-trioxides are bench-stable and easy-to-use reagents. In photoredox catalysis, they generate methyl and perdeuteromethyl radicals which can add to a variety of radical acceptors, including olefins, acrylamides, quinoxalinones, isocyanides, enol silanes, and N-Ts acrylamide. As byproduct, a salt is formed which can be regenerated to the original methylating agent. Flow chemistry provides an option for reaction scale-up further underscoring the synthetic usefulness of these methylation reagents. Mechanistic investigations suggest a single-electron transfer (SET) pathway induced by photoredox catalysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
26.60
自引率
6.60%
发文量
3549
审稿时长
1.5 months
期刊介绍:
Angewandte Chemie, a journal of the German Chemical Society (GDCh), maintains a leading position among scholarly journals in general chemistry with an impressive Impact Factor of 16.6 (2022 Journal Citation Reports, Clarivate, 2023). Published weekly in a reader-friendly format, it features new articles almost every day. Established in 1887, Angewandte Chemie is a prominent chemistry journal, offering a dynamic blend of Review-type articles, Highlights, Communications, and Research Articles on a weekly basis, making it unique in the field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


