3-(3-indolomethyl)-oxindoles 的不对称催化简易合成,用于构建三叉类似物
IF 4.3
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用一种 N,N'-二氧/Ho(III)络合物,通过将吲哚取代的烯醇化酮酯与 3-溴-3-取代的吲哚加成,实现了 3-(3-吲哚甲基)吲哚的不对称合成。研究人员获得了多种可能具有生物活性的 3-(3-indolomethyl)羰基吲哚,其产率高,非对映和对映选择性高(产率高达 97%,对映选择性为 19:1,ee 为 98%)。此外,非对映选择性的时间依赖性逆转使得获得光学活性二元酯成为可能。该产品随后的简便转化为三叉类似物提供了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
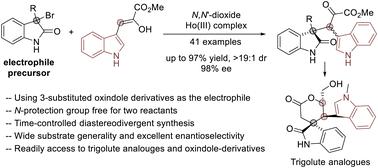
Asymmetric catalytic concise synthesis of 3-(3-indolomethyl)-oxindoles for the construction of trigolute analogs†
Asymmetric synthesis of 3-(3-indolomethyl)oxindoles through the addition of indole-substituted enolized ketoesters to 3-bromo-3-substituted oxindoles has been achieved using a N,N′-dioxide/Ho(iii) complex. A number of 3-(3-indolomethyl)oxindoles, which may possess biological activity, were obtained in good yields with high diastereo- and enantioselectivities (up to 97% yield, >19 : 1 dr, 98% ee). Furthermore, time-dependent reversal of diastereoselectivity enabled access to optically active diastereomers. The product followed by facile transformations gave a new route into trigolute analogs.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Communications
化学-化学综合
CiteScore
8.60
自引率
4.10%
发文量
2705
审稿时长
1.4 months
期刊介绍:
ChemComm (Chemical Communications) is renowned as the fastest publisher of articles providing information on new avenues of research, drawn from all the world''s major areas of chemical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


