卵巢癌免疫疗法:迈向基于免疫表型的定制方法
IF 81.1
1区 医学
Q1 ONCOLOGY
引用次数: 0
摘要
尽管有文献证明卵巢癌细胞表达免疫检查点分子(如 PD-1 和 PD-L1),而且肿瘤浸润淋巴细胞的存在与该肿瘤类型患者良好的总体生存结果之间存在正相关,但迄今为止在这些患者中测试免疫检查点抑制剂(ICIs)的试验结果却令人失望。对 ICIs 缺乏反应可归因于肿瘤异质性以及与肿瘤微环境(TME)相关的固有或获得性抵抗。了解肿瘤免疫生物学、发现用于患者选择的生物标志物以及建立最佳治疗组合仍然是卵巢癌免疫疗法未来应用的希望所在,但也是关键挑战。在本综述中,我们总结了在卵巢癌患者中测试 ICIs 的试验结果。我们建议对这种恶性肿瘤进行系统的基于 CD8+ T 细胞的免疫分型分类,然后讨论临床前数据,为用联合免疫疗法治疗这种免疫分型提供依据。我们认为,将准确的 TME 免疫表型特征与基因数据相结合,可以设计出量身定制的治疗方法,并改善临床试验中的患者招募情况。最后,我们提出了一个路线图,将基于组织的特征分析纳入其中,以指导未来测试收养细胞疗法方法的试验,并评估新型免疫疗法组合,同时促进合作研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Immunotherapy for ovarian cancer: towards a tailored immunophenotype-based approach
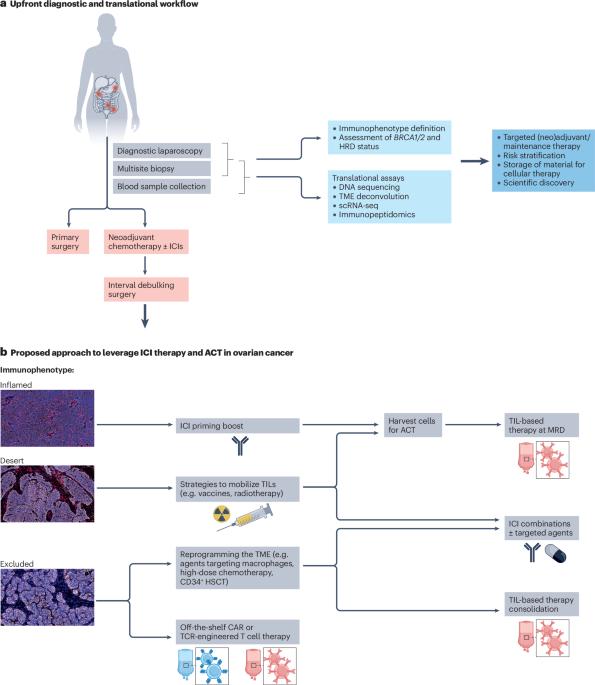
Despite documented evidence that ovarian cancer cells express immune-checkpoint molecules, such as PD-1 and PD-L1, and of a positive correlation between the presence of tumour-infiltrating lymphocytes and favourable overall survival outcomes in patients with this tumour type, the results of trials testing immune-checkpoint inhibitors (ICIs) in these patients thus far have been disappointing. The lack of response to ICIs can be attributed to tumour heterogeneity as well as inherent or acquired resistance associated with the tumour microenvironment (TME). Understanding tumour immunobiology, discovering biomarkers for patient selection and establishing optimal treatment combinations remains the hope but also a key challenge for the future application of immunotherapy in ovarian cancer. In this Review, we summarize results from trials testing ICIs in patients with ovarian cancer. We propose the implementation of a systematic CD8+ T cell-based immunophenotypic classification of this malignancy, followed by discussions of the preclinical data providing the basis to treat such immunophenotypes with combination immunotherapies. We posit that the integration of an accurate TME immunophenotype characterization with genetic data can enable the design of tailored therapeutic approaches and improve patient recruitment in clinical trials. Lastly, we propose a roadmap incorporating tissue-based profiling to guide future trials testing adoptive cell therapy approaches and assess novel immunotherapy combinations while promoting collaborative research. Although ovarian cancer is considered an immunoreactive disease, moderate to no efficacy has been shown in the trials testing immune-checkpoint inhibitors in these patients performed thus far. The authors of this Review summarize these results and propose a systematic classification of ovarian cancer based on CD8+ T cell immunophenotypes that, integrated with genetic data, can enable the design of tailored therapeutic approaches, including adoptive cell therapy and novel immunotherapy combinations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
99.40
自引率
0.40%
发文量
114
审稿时长
6-12 weeks
期刊介绍:
Nature Reviews publishes clinical content authored by internationally renowned clinical academics and researchers, catering to readers in the medical sciences at postgraduate levels and beyond. Although targeted at practicing doctors, researchers, and academics within specific specialties, the aim is to ensure accessibility for readers across various medical disciplines. The journal features in-depth Reviews offering authoritative and current information, contextualizing topics within the history and development of a field. Perspectives, News & Views articles, and the Research Highlights section provide topical discussions, opinions, and filtered primary research from diverse medical journals.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


