CsPbBr3 纳米晶体的化学不稳定性以及合成前体驱动的 CsPbBr3 和 Cs4PbBr6 纳米晶体之间的可逆转变
IF 7.2
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
过氧化物纳米晶体因其独特的光电特性而日益受到关注。然而,由于其离子性质,其化学稳定性相对较差。在这项工作中,我们发现在常温下合成 CsPbBr3 纳米晶体(NCs)时使用的无机阳离子(如 Cs+)和配体(如十二烷基二甲基溴化铵,DDAB)会在合成过程中或合成后的处理过程中迅速将 CsPbBr3 NCs 转化为 Cs4PbBr6 NCs。这些阳离子(Cs+、DDA+)参与了 CsPbBr3 NCs 的化学组成和解离。然而,由于 DDA+ 与 Cs+ 的性质不同,DDA+ 除了诱导生成 Cs4PbBr6 NCs 之外,还诱导生成了一种杂质。光学光谱和透射电子显微镜观察了这一转变过程。只要在 Cs4PbBr6 NCs 中加入足量的 PbBr2,就能将 Cs4PbBr6 NCs 完全反向转化为 CsPbBr3 NCs。因此,正向和逆向反应是由 Cs+ 和 Pb2+ 驱动的。在反向转化过程中加入 DDAB 进行钝化,所得到的产物的光量子产率(PLQY,∼90%)比未加入 DDAB 的产物(∼69%)更高。将 Cs4PbBr6 NCs 视为铯源,反向转化可类比于 CsPbBr3 NCs 的合成,为在室温下合成具有理论化学计量的发光包晶 NCs 提供了一种可能的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
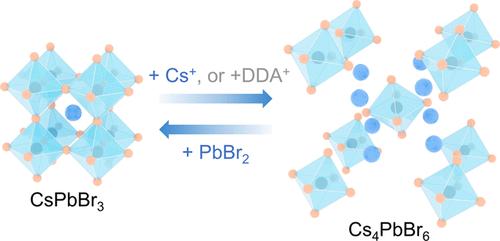
Chemical Instability of CsPbBr3 Nanocrystals and the Reversible Transformation between CsPbBr3 and Cs4PbBr6 Nanocrystals as Driven by Synthetic Precursors
Perovskite nanocrystals attract growing interest owing to their unique optoelectronic properties. However, their chemical stability is relatively poor due to their ionic nature. In this work, we found that inorganic cations (e.g., Cs+) and ligands (e.g., didodecyldimethylammonium bromide, DDAB), used in the synthesis of CsPbBr3 nanocrystals (NCs) at ambient temperature, quickly converted CsPbBr3 NCs to Cs4PbBr6 NCs during the synthesis or postsynthetic treatment. These cations (Cs+, DDA+) were involved in both the chemical composition and dissociation of CsPbBr3 NCs. Nevertheless, DDA+ induced the generation of an impurity in addition to Cs4PbBr6 NCs due to its different nature from that of Cs+. The transformation process was observed by optical spectroscopy and transmission electron microscopy. The reverse transformation of Cs4PbBr6 NCs to CsPbBr3 NCs can be carried out completely by adding sufficient PbBr2 into Cs4PbBr6 NCs. Therefore, the forward and backward reactions were driven by Cs+ and Pb2+. With additional DDAB for passivation during the reverse transformation, the resulting product possessed a better photoluminescence quantum yield (PLQY, ∼90%) compared with that without the involvement of DDAB (∼69%). By considering the Cs4PbBr6 NCs as the Cs source, the reverse transformation can be analogized to the synthesis of CsPbBr3 NCs, providing a possible strategy for synthesizing luminescent perovskite NCs at room temperature with theoretical stoichiometry.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


