在黄素和碘的催化下,通过 C-H 键活化形成有氧氧化 C-C 键。
IF 2.9
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
摘要
我们报告了一种在黄素和碘催化下利用 sp3 C-H 键活化四氢异喹啉的无金属/无光有氧氧化 C-C 键形成的方法。该双催化系统通过两个 sp3 C-H 键之间的交叉脱氢偶联实现了氧化曼尼希反应和氮杂亨利反应。此外,黄素-碘耦合催化还被应用于吡咯并[2,1-a]异喹啉类化合物的合成,即通过连续的氧化-1,3-二极环加成和脱氢芳香化反应合成吡咯并[2,1-a]异喹啉类化合物。仿生黄素催化能有效激活分子氧,因此非金属双催化系统能利用分子氧作为环境友好型末端氧化剂进行绿色氧化转化,并生成良性水。本文章由计算机程序翻译,如有差异,请以英文原文为准。
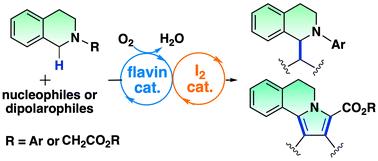
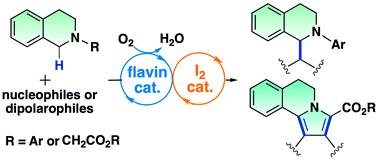
Aerobic oxidative C–C bond formation through C–H bond activation catalysed by flavin and iodine†
We report a metal/light-free aerobic oxidative C–C bond formation using sp3 C–H bond activation of tetrahydroisoquinolines catalyzed by flavin and iodine. The dual catalytic system enabled the oxidative Mannich and aza-Henry reactions by the cross-dehydrogenative coupling between two sp3 C–H bonds. Furthermore, the flavin–iodine-coupled catalysis was applied to the synthesis of pyrrolo[2,1-a]isoquinolines through the sequential oxidative 1,3-dipolar cycloaddition and dehydrogenative aromatization. The biomimetic flavin catalysis efficiently activates molecular oxygen; thus the non-metal dual catalytic system enables green oxidative transformation using molecular oxygen as an environmentally friendly terminal oxidant which generates benign water.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


