平衡超分子晶体中氢的体积容量和重量容量
IF 20.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
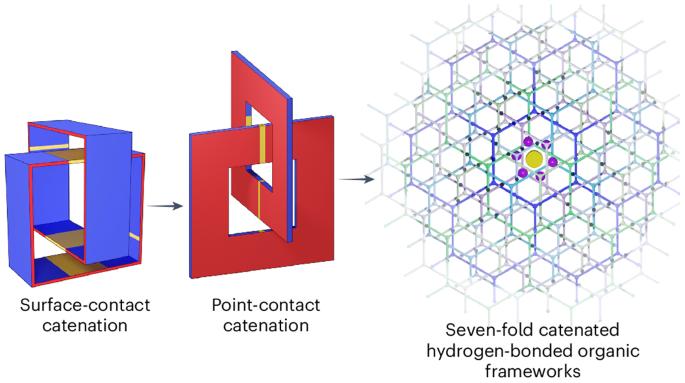
氢的储存是其应用的关键。开发具有高容积和重力储存能力的吸附材料具有挑战性,而这两种能力对于高效利用氢气作为燃料至关重要。在此,我们报告了氢键有机框架(RP-H100 和 RP-H101)中的受控催化策略,该策略依赖于多个氢键以点接触的方式引导催化,从而产生高体积表面积和重力表面积、坚固性和理想的储氢孔径(~1.2-1.9 nm)。这种方法是将九个咪唑annulated三庚烯六面体组装成一个二级六边形上层结构,该结构包含三个开放通道,其中七个六面体通过这些通道相互渗透,形成一个七重猫式上层结构。RP-H101 在组合温度和压力摆动(77 K/100 bar → 160 K/5 bar)条件下显示出较高的氢气容积输送能力(53.7 g l-1)和重量输送能力(9.3 wt%)。这项工作说明了超分子晶体作为储氢候选材料的优点。本文章由计算机程序翻译,如有差异,请以英文原文为准。

Balancing volumetric and gravimetric capacity for hydrogen in supramolecular crystals
The storage of hydrogen is key to its applications. Developing adsorbent materials with high volumetric and gravimetric storage capacities, both of which are essential for the efficient use of hydrogen as a fuel, is challenging. Here we report a controlled catenation strategy in hydrogen-bonded organic frameworks (RP-H100 and RP-H101) that depends on multiple hydrogen bonds to guide catenation in a point-contact manner, resulting in high volumetric and gravimetric surface areas, robustness and ideal pore diameters (~1.2–1.9 nm) for hydrogen storage. This approach involves assembling nine imidazole-annulated triptycene hexaacids into a secondary hexagonal superstructure containing three open channels through which seven of the hexagons interpenetrate to form a seven-fold catenated superstructure. RP-H101 exhibits high deliverable volumetric (53.7 g l−1) and gravimetric (9.3 wt%) capacities for hydrogen under a combined temperature and pressure swing (77 K/100 bar → 160 K/5 bar). This work illustrates the virtues of supramolecular crystals as promising candidates for hydrogen storage. The development of materials for efficient hydrogen storage is desirable. Now, hydrogen-bonded organic frameworks exhibiting both high volumetric and gravimetric hydrogen storage capacities have been synthesized; hydrogen-bonding interactions are key to guide the catenation of the structure, effectively minimizing the surface area loss in the supramolecular crystals.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


