人类眶额叶皮层单核转录组特征分析揭示了衰老和精神疾病的趋同效应
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
摘要
衰老是一个复杂的生物过程,也是神经退行性疾病的最大风险因素。患有精神疾病的人患神经退行性疾病的风险也会增加。在这里,我们通过对 87 名患有和未患有精神疾病的患者的眶额叶皮层约 80 万个细胞核进行分析,描述了大脑中与年龄相关的转录组变化,并在一个包含 32 名患者的独立队列中复制了研究结果。衰老对所有细胞类型都有影响,其中LAMP5+LHX6+中间神经元是灵长类动物中最多的细胞类型,受影响最大。突触传递的中断是衰老组织中受影响最大的途径。与年龄相关的转录组变化与在阿尔茨海默病中观察到的多种细胞类型的变化相重叠。我们发现了精神障碍患者转录组加速衰老的证据,并在多种细胞类型中展示了衰老和精神病理学的交汇特征。我们的研究结果揭示了细胞类型的特异性效应和年龄相关变化的生物通路,以及它们与精神病诊断驱动的效应的趋同性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
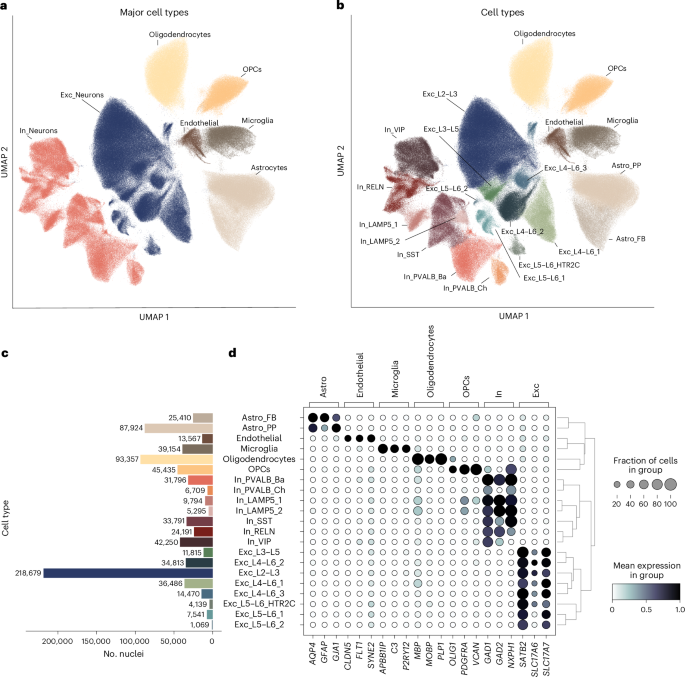
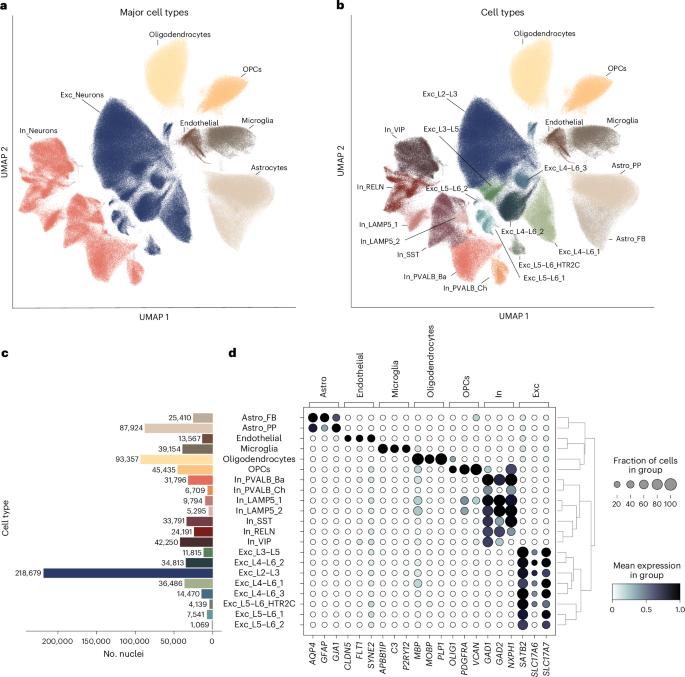
Single-nucleus transcriptomic profiling of human orbitofrontal cortex reveals convergent effects of aging and psychiatric disease
Aging is a complex biological process and represents the largest risk factor for neurodegenerative disorders. The risk for neurodegenerative disorders is also increased in individuals with psychiatric disorders. Here, we characterized age-related transcriptomic changes in the brain by profiling ~800,000 nuclei from the orbitofrontal cortex from 87 individuals with and without psychiatric diagnoses and replicated findings in an independent cohort with 32 individuals. Aging affects all cell types, with LAMP5+LHX6+ interneurons, a cell-type abundant in primates, by far the most affected. Disrupted synaptic transmission emerged as a convergently affected pathway in aged tissue. Age-related transcriptomic changes overlapped with changes observed in Alzheimer’s disease across multiple cell types. We find evidence for accelerated transcriptomic aging in individuals with psychiatric disorders and demonstrate a converging signature of aging and psychopathology across multiple cell types. Our findings shed light on cell-type-specific effects and biological pathways underlying age-related changes and their convergence with effects driven by psychiatric diagnosis. Single-cell profiling in the human cortex reveals aging-associated transcriptomic changes across all brain cell types, which overlap with effects with Alzheimer’s disease and show a convergent signature with psychopathology across multiple cell types.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


