抗 NMDAR 自身免疫性脑炎的结构和功能机制
IF 12.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
针对神经元膜蛋白的自身抗体可表现为自身免疫性脑炎,诱发癫痫发作、认知功能障碍和精神病。抗 N-甲基-d-天冬氨酸受体(NMDAR)脑炎是最主要的自身免疫性脑炎;然而,对自身抗体如何识别和改变受体功能的了解仍然有限。在这里,我们使用单颗粒电子冰冻显微镜测定了人类和大鼠 NMDAR 与三种不同的患者衍生抗体结合的结构。这些抗体结合了 GluN1 亚基氨基末端结构域的不同区域。通过电生理学研究,我们发现这三种自身抗体都会急性地直接降低原发性神经元中 NMDAR 通道的功能。抗体显示出不同的结合和抗体-受体复合物形成的配比,其中一种抗体(003-102)还导致 NMDAR 的突触定位减少。这些研究证明了抗 NMDAR 自身抗体在自身免疫性脑炎中的表位识别和直接通道调节机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。
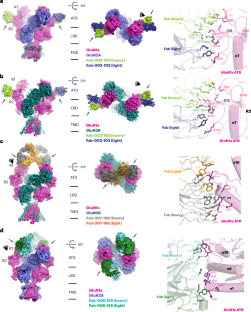

Structural and functional mechanisms of anti-NMDAR autoimmune encephalitis
Autoantibodies against neuronal membrane proteins can manifest in autoimmune encephalitis, inducing seizures, cognitive dysfunction and psychosis. Anti-N-methyl-d-aspartate receptor (NMDAR) encephalitis is the most dominant autoimmune encephalitis; however, insights into how autoantibodies recognize and alter receptor functions remain limited. Here we determined structures of human and rat NMDARs bound to three distinct patient-derived antibodies using single-particle electron cryo-microscopy. These antibodies bind different regions within the amino-terminal domain of the GluN1 subunit. Through electrophysiology, we show that all three autoantibodies acutely and directly reduced NMDAR channel functions in primary neurons. Antibodies show different stoichiometry of binding and antibody–receptor complex formation, which in one antibody, 003-102, also results in reduced synaptic localization of NMDARs. These studies demonstrate mechanisms of diverse epitope recognition and direct channel regulation of anti-NMDAR autoantibodies underlying autoimmune encephalitis. Anti-NMDA receptor encephalitis is the most common autoimmune encephalitis. Michalski et al. reveal epitope diversity, conformational changes and functional impacts of the autoantibodies using cryo-EM and electrophysiology.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Structural & Molecular Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-BIOPHYSICS
CiteScore
22.00
自引率
1.80%
发文量
160
审稿时长
3-8 weeks
期刊介绍:
Nature Structural & Molecular Biology is a comprehensive platform that combines structural and molecular research. Our journal focuses on exploring the functional and mechanistic aspects of biological processes, emphasizing how molecular components collaborate to achieve a particular function. While structural data can shed light on these insights, our publication does not require them as a prerequisite.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


