金属酶中质子耦合电子转移的构象控制。
IF 38.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
从二氮的还原到水的氧化,金属酶催化的化学变化是全球地球化学和生物化学循环的基础。这些反应是已知的动力学和热力学上最具挑战性的过程,需要对自然界的基本组成单元--电子和质子--进行复杂的编排,以达到最高的精确度和准确性。许多金属酶催化作用的决定速率步骤包括蛋白质结构的重新排列,这表明大自然已经进化到可以利用蛋白质分子结构的宏观变化来控制金属因子电子结构的亚原子变化。氮化酶、光系统 II 和核糖核苷酸还原酶中的质子耦合电子传递机制就是分子结构和电子结构控制之间相互作用的例证。我们介绍了数十年来对上述每个系统的研究成果,并阐明了这些金属酶连接蛋白的结构变化与功能结果之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
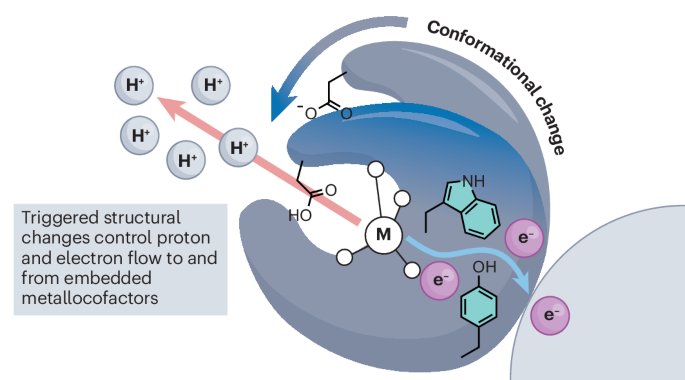
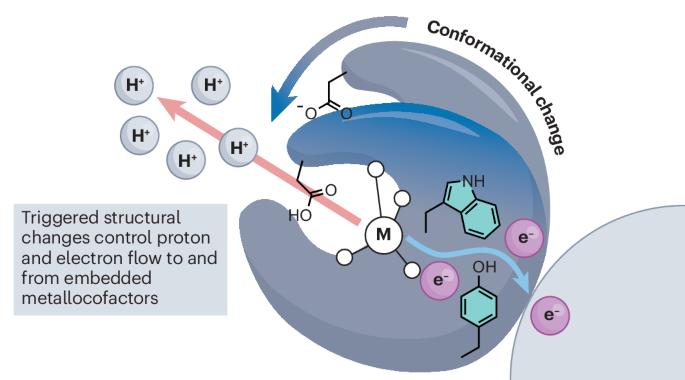
Conformational control over proton-coupled electron transfer in metalloenzymes
From the reduction of dinitrogen to the oxidation of water, the chemical transformations catalysed by metalloenzymes underlie global geochemical and biochemical cycles. These reactions represent some of the most kinetically and thermodynamically challenging processes known and require the complex choreography of the fundamental building blocks of nature, electrons and protons, to be carried out with utmost precision and accuracy. The rate-determining step of catalysis in many metalloenzymes consists of a protein structural rearrangement, suggesting that nature has evolved to leverage macroscopic changes in protein molecular structure to control subatomic changes in metallocofactor electronic structure. The proton-coupled electron transfer mechanisms operative in nitrogenase, photosystem II and ribonucleotide reductase exemplify this interplay between molecular and electronic structural control. We present the culmination of decades of study on each of these systems and clarify what is known regarding the interplay between structural changes and functional outcomes in these metalloenzyme linchpins. Rate-limiting conformational changes often gate the formation of catalytically active metalloenzyme states. We review examples of the interplay between macroscopic changes in protein molecular structure and subatomic changes in metallocofactor electronic structure that together enable precision control over nature’s redox machines.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature reviews. Chemistry
Chemical Engineering-General Chemical Engineering
CiteScore
52.80
自引率
0.80%
发文量
88
期刊介绍:
Nature Reviews Chemistry is an online-only journal that publishes Reviews, Perspectives, and Comments on various disciplines within chemistry. The Reviews aim to offer balanced and objective analyses of selected topics, providing clear descriptions of relevant scientific literature. The content is designed to be accessible to recent graduates in any chemistry-related discipline while also offering insights for principal investigators and industry-based research scientists. Additionally, Reviews should provide the authors' perspectives on future directions and opinions regarding the major challenges faced by researchers in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


