锰(I)催化的 C(sp3)-H 键与芳基异氰酸酯的直接加成。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-03
DOI:10.1021/acs.joc.4c01581
引用次数: 0
摘要
在过渡金属催化下,异氰酸酯与 C-H 键的加成是一种非常有效的方法,可提供具有合成和生物重要意义的酰胺。然而,底物仅限于 C(sp2)-H 键。在这项工作中,开发了一种由锰(I)催化的 8-甲基喹啉的 C(sp3)-H 键与芳基异氰酸酯的高效直接加成反应,从而以中等至高产率合成了各种 α-喹啉基酰胺化合物。该反应具有广泛的底物范围和良好的官能团耐受性。根据实验结果提出了一种可能的机理。本文章由计算机程序翻译,如有差异,请以英文原文为准。
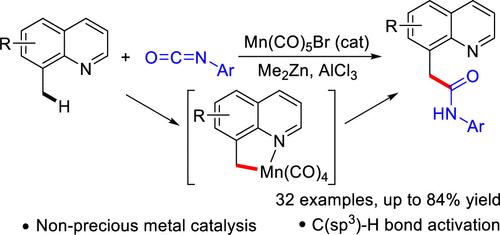
Manganese(I)-Catalyzed Direct Addition of C(sp3)-H Bonds to Aryl Isocyanates.
The addition of C-H bonds to isocyanates catalyzed by transition metals is a highly auspicious methodology for providing synthetically and biologically important amides. However, the substrates are limited to C(sp2)-H bonds. In this work, an efficient manganese(I)-catalyzed direct addition reaction of C(sp3)-H bonds of 8-methylquinolines to aryl isocyanates has been developed, leading to the synthesis of various α-quinolinyl amide compounds in moderate to high yields. The reaction has a broad range of substrates and a good functional group tolerance. A possible mechanism is proposed based on the experimental results.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


