手性萘酚催化的烯基和芳基硼酸与 α、β-不饱和环 N-磺酰基酮亚胺的对映选择性共轭加成。
IF 3.3
2区 化学
Q1 CHEMISTRY, ORGANIC
The Journal of Organic Chemistry
Pub Date : 2024-09-20
Epub Date: 2024-09-02
DOI:10.1021/acs.joc.4c01269
引用次数: 0
摘要
本研究报道了手性二萘酚催化的烯基硼酸和杂芳基硼酸与环状 N-磺酰基酮亚胺的对映体选择性共轭加成,可提供高产率和中等到极好的对映体选择性(高达 >99% ee)的 1,4 加成产物。这种温和、可扩展的催化系统具有高效率和广泛的底物范围。此外,在更苛刻的条件下,芳基硼酸也是可行的亲核体。本文章由计算机程序翻译,如有差异,请以英文原文为准。
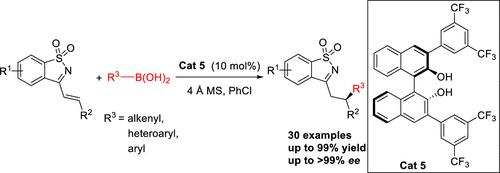
Chiral Binaphthol-Catalyzed Enantioselective Conjugate Addition of Alkenyl and Arylboronic Acids to α,β-Unsaturated Cyclic N-Sulfonyl Ketimines.
The chiral binaphthol-catalyzed enantioselective conjugate addition of alkenylboronic acids and heteroarylboronic acids to cyclic N-sulfonyl ketimines is reported, providing the 1,4-addition products in high yields and moderate to excellent enantioselectivities (up to >99% ee). This mild, scalable catalytic system exhibits high efficiency and broad substrate scopes. Additionally, arylboronic acids were viable nucleophiles under more forcing conditions.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

The Journal of Organic Chemistry
化学-有机化学
CiteScore
6.20
自引率
11.10%
发文量
1467
审稿时长
2 months
期刊介绍:
The Journal of Organic Chemistry welcomes original contributions of fundamental research in all branches of the theory and practice of organic chemistry. In selecting manuscripts for publication, the editors place emphasis on the quality and novelty of the work, as well as the breadth of interest to the organic chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


