未活化烯烃的无过渡金属双官能化:芳基化/唑化、芳基化/氯化和芳基化/氰化
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
芳基乙胺是药物化合物中的一种特殊支架,是许多医疗药物(包括用于治疗神经系统疾病和疼痛的药物)的骨架。它们的生物医学意义激发了新的合成方法,这些方法依赖于过渡金属催化的烯烃氨基芳基化反应,通常与光氧化催化剂或光敏剂一起进行,并由定向或稳定基团引导。在此,我们介绍一种在无过渡金属条件下对未活化烯烃进行叠氮芳基化的简单而有效的方法。用可见光或近紫外光照射现成的三芳基二氯化铋,会产生一个芳基自由基,选择性地与烯烃发生加成反应,生成的均苄基自由基会被等效的胺截获。这种方法提供了广泛的底物范围,还可以对未活化的烯烃进行芳基氯化和芳基氰化。本文章由计算机程序翻译,如有差异,请以英文原文为准。
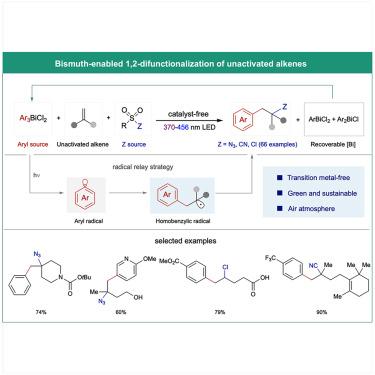
Transition metal-free difunctionalization of unactivated alkenes: Arylation/azidation, arylation/chlorination, and arylation/cyanation
Arylethylamines represent a privileged scaffold in pharmaceutical compounds and form the backbone of many medical drugs, including those used for treating neurological diseases and pain. Their biomedical significance has inspired new synthetic methods that rely on transition metal-catalyzed aminoarylation reaction to an alkene, often in conjunction with a photoredox catalyst or a photosensitizer and guided by a directing or stabilizing group. Here, we introduce a simple and effective method for the azidoarylation of unactivated alkenes under transition metal-free conditions. Visible- or near-UV-light irradiation of readily available triarylbismuth dichlorides generates an aryl radical that selectively adds to the alkene, and the resulting homobenzyl radical is intercepted by an amine equivalent. This method offers a broad substrate scope and also enables the arylchlorination and arylcyanation of unactivated alkenes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


