蛋白质淀粉样蛋白对表型信息的非规范遗传
IF 17.3
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
摘要
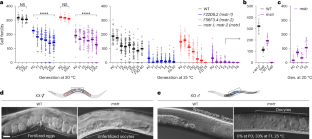
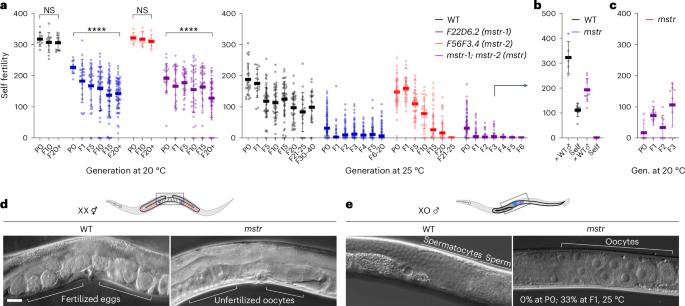
所有已知的动物遗传表型信息都是通过直接遗传核酸、核酸的共价修饰或组蛋白修饰来传递的,这些修饰可调节相关基因组区域的表达。然而,许多家族性状和疾病并不能归因于已知的遗传分子因素。在这里,我们确定了在野生型动物中稳定遗传并影响性状的淀粉样蛋白结构。通过遗传、环境或药物治疗对其进行干扰会导致发育表型,并通过表观遗传传递给后代。将从不同表型背景中分离出的淀粉样蛋白注射到幼稚动物体内,可重现后代的相关表型。遗传学和蛋白质组学分析表明,26S 蛋白酶体及其保守的调控因子可维持淀粉样蛋白的跨代遗传,从而实现生殖细胞的正常性别分化。我们认为,蛋白质表观遗传记忆的遗传协调了发育时间和模式与环境之间的关系,从而赋予了适应能力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Noncanonical inheritance of phenotypic information by protein amyloids
All known heritable phenotypic information in animals is transmitted by direct inheritance of nucleic acids, their covalent modifications or histone modifications that modulate expression of associated genomic regions. Nonetheless, numerous familial traits and disorders cannot be attributed to known heritable molecular factors. Here we identify amyloid-like protein structures that are stably inherited in wild-type animals and influence traits. Their perturbation by genetic, environmental or pharmacological treatments leads to developmental phenotypes that can be epigenetically passed onto progeny. Injection of amyloids isolated from different phenotypic backgrounds into naive animals recapitulates the associated phenotype in offspring. Genetic and proteomic analyses reveal that the 26S proteasome and its conserved regulators maintain heritable amyloids across generations, which enables proper germ cell sex differentiation. We propose that inheritance of a proteinaceous epigenetic memory coordinates developmental timing and patterning with the environment to confer adaptive fitness. Eroglu et al. describe protein amyloid structures that are stably inherited across generations and transmit epigenetic memory in Caenorhabditis elegans. MSTR protein loss results in a transgenerational feminization phenotype through ectopic GLD-1 expression.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
Nature Cell Biology
生物-细胞生物学
CiteScore
28.40
自引率
0.90%
发文量
219
审稿时长
3 months
期刊介绍:
Nature Cell Biology, a prestigious journal, upholds a commitment to publishing papers of the highest quality across all areas of cell biology, with a particular focus on elucidating mechanisms underlying fundamental cell biological processes. The journal's broad scope encompasses various areas of interest, including but not limited to:
-Autophagy
-Cancer biology
-Cell adhesion and migration
-Cell cycle and growth
-Cell death
-Chromatin and epigenetics
-Cytoskeletal dynamics
-Developmental biology
-DNA replication and repair
-Mechanisms of human disease
-Mechanobiology
-Membrane traffic and dynamics
-Metabolism
-Nuclear organization and dynamics
-Organelle biology
-Proteolysis and quality control
-RNA biology
-Signal transduction
-Stem cell biology
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


