表观遗传疗法可促进转座元件转录,从而在胶质母细胞瘤细胞中产生肿瘤富集抗原
IF 31.7
1区 生物学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
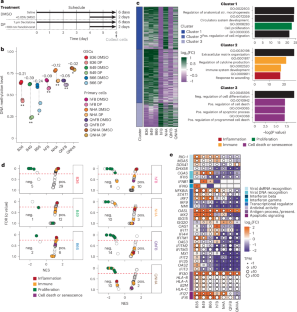
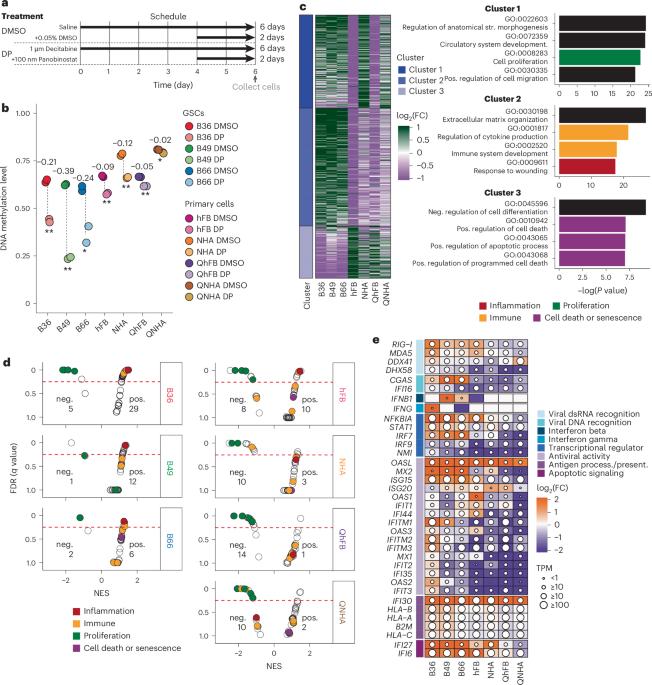
抑制表观遗传调节剂可以转录重新激活转座元件(TE)。这些TE转录本通常会产生独特的多肽,可作为免疫疗法的免疫原性抗原。胶质母细胞瘤是一种侵袭性脑癌,其突变和新抗原负担较低。我们用表观遗传学药物处理了源自患者的原代胶质母细胞瘤干细胞系、星形胶质细胞系和原代成纤维细胞系,并确定了治疗诱导的、在癌细胞中优先表达的 TE 衍生转录本。我们利用液相色谱-串联质谱下拉实验验证了这些转录本能产生人类白细胞抗原 I 类呈递抗原。重要的是,在表观遗传学治疗后,许多 TEs 也被转录,甚至在增殖的非肿瘤细胞系中也是如此,这表明 CRISPR 介导的激活等靶向策略可以最大限度地减少激活不需要的基因组区域的潜在副作用。这些结果凸显了利用治疗诱导的 TE 衍生抗原进行靶向免疫疗法的谨慎性和未来转化工作的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Epigenetic therapy potentiates transposable element transcription to create tumor-enriched antigens in glioblastoma cells
Inhibiting epigenetic modulators can transcriptionally reactivate transposable elements (TEs). These TE transcripts often generate unique peptides that can serve as immunogenic antigens for immunotherapy. Here, we ask whether TEs activated by epigenetic therapy could appreciably increase the antigen repertoire in glioblastoma, an aggressive brain cancer with low mutation and neoantigen burden. We treated patient-derived primary glioblastoma stem cell lines, an astrocyte cell line and primary fibroblast cell lines with epigenetic drugs, and identified treatment-induced, TE-derived transcripts that are preferentially expressed in cancer cells. We verified that these transcripts could produce human leukocyte antigen class I-presented antigens using liquid chromatography with tandem mass spectrometry pulldown experiments. Importantly, many TEs were also transcribed, even in proliferating nontumor cell lines, after epigenetic therapy, which suggests that targeted strategies like CRISPR-mediated activation could minimize potential side effects of activating unwanted genomic regions. The results highlight both the need for caution and the promise of future translational efforts in harnessing treatment-induced TE-derived antigens for targeted immunotherapy. Treatment of primary glioblastoma cell lines with epigenetic therapy reactivates transposable elements (TEs). TE-derived transcripts can produce human leukocyte antigen class I-presented antigens, which could potentially be therapeutically targeted.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature genetics
生物-遗传学
CiteScore
43.00
自引率
2.60%
发文量
241
审稿时长
3 months
期刊介绍:
Nature Genetics publishes the very highest quality research in genetics. It encompasses genetic and functional genomic studies on human and plant traits and on other model organisms. Current emphasis is on the genetic basis for common and complex diseases and on the functional mechanism, architecture and evolution of gene networks, studied by experimental perturbation.
Integrative genetic topics comprise, but are not limited to:
-Genes in the pathology of human disease
-Molecular analysis of simple and complex genetic traits
-Cancer genetics
-Agricultural genomics
-Developmental genetics
-Regulatory variation in gene expression
-Strategies and technologies for extracting function from genomic data
-Pharmacological genomics
-Genome evolution
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


