逆转录转座子成瘾通过表观遗传激活的小RNA促进中心粒功能
IF 15.8
1区 生物学
Q1 PLANT SCIENCES
引用次数: 0
摘要
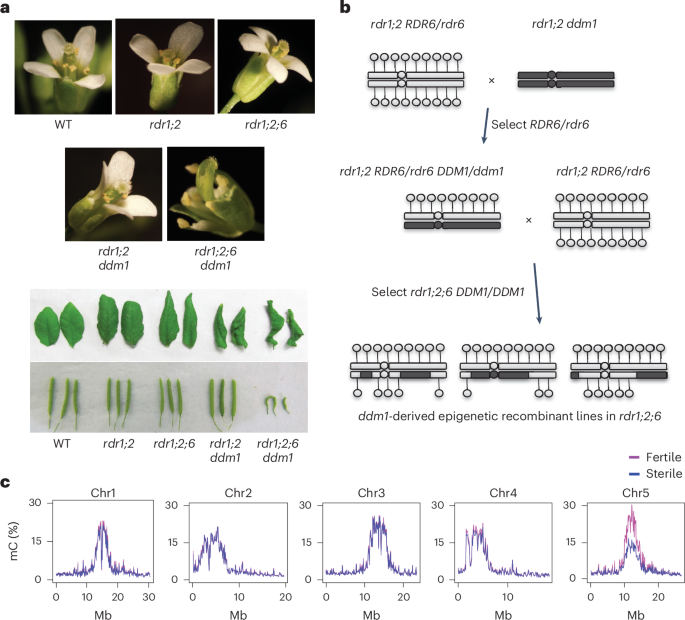
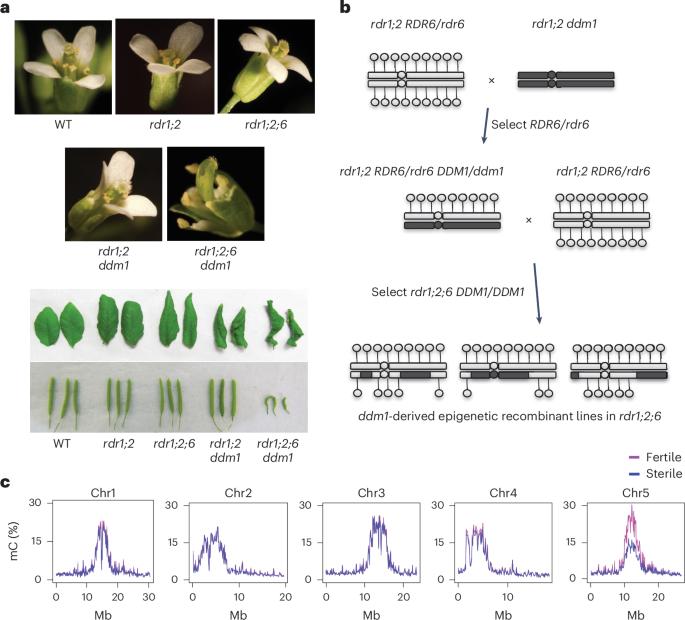
逆转座子在重复扩增和清除的循环中侵入了真核生物的中心粒,但中心粒逆转座子的功能仍不清楚。在拟南芥中,中心粒ATHILA逆转座子在DNA甲基化减少1(DDM1)突变体中产生表观遗传激活的短干扰RNA。在这里,我们发现同时失去 DDM1 和 RNA 依赖性 RNA 聚合酶的突变体具有多向发育缺陷,并在有丝分裂过程中出现 5 号染色体的错误分离。生育力和分离缺陷与中心粒5具有表观遗传性,可以通过将人工小RNA引导至ATHILA5逆转录载体来挽救,ATHILA5逆转录载体会中断串联卫星重复序列。表观遗传激活的短干扰 RNA 可促进有丝分裂中的中心粒周围凝聚、染色体内聚和染色体分离。我们认为 ATHILA 的插入会抑制中心粒的转录,同时在缺乏 DDM1 的情况下,使中心粒的功能依赖于逆转座小 RNA。这与裂殖酵母(Schizosaccharomyces pombe)和人类的情况相似,前者的染色体内聚依赖于 RNA 干扰,后者的染色体分离依赖于 RNA 干扰和 HELLSDDM1。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Retrotransposon addiction promotes centromere function via epigenetically activated small RNAs
Retrotransposons have invaded eukaryotic centromeres in cycles of repeat expansion and purging, but the function of centromeric retrotransposons has remained unclear. In Arabidopsis, centromeric ATHILA retrotransposons give rise to epigenetically activated short interfering RNAs in mutants in DECREASE IN DNA METHYLATION1 (DDM1). Here we show that mutants that lose both DDM1 and RNA-dependent RNA polymerase have pleiotropic developmental defects and mis-segregate chromosome 5 during mitosis. Fertility and segregation defects are epigenetically inherited with centromere 5, and can be rescued by directing artificial small RNAs to ATHILA5 retrotransposons that interrupt tandem satellite repeats. Epigenetically activated short interfering RNAs promote pericentromeric condensation, chromosome cohesion and chromosome segregation in mitosis. We propose that insertion of ATHILA silences centromeric transcription, while simultaneously making centromere function dependent on retrotransposon small RNAs in the absence of DDM1. Parallels are made with the fission yeast Schizosaccharomyces pombe, where chromosome cohesion depends on RNA interference, and with humans, where chromosome segregation depends on both RNA interference and HELLSDDM1. Centromeric satellite repeats on Arabidopsis chromosome 5 are interrupted by ATHILA5 retrotransposons, and cohesion is compromised in ddm1 chromatin remodelling mutants that have also lost RNAi. Mis-segregation is epigenetically inherited but can be rescued by ATHILA5 small RNA.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Plants
PLANT SCIENCES-
CiteScore
25.30
自引率
2.20%
发文量
196
期刊介绍:
Nature Plants is an online-only, monthly journal publishing the best research on plants — from their evolution, development, metabolism and environmental interactions to their societal significance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


