肿瘤衍生的细胞外小囊泡是治疗用纳米粒子输送的屏障
IF 38.5
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
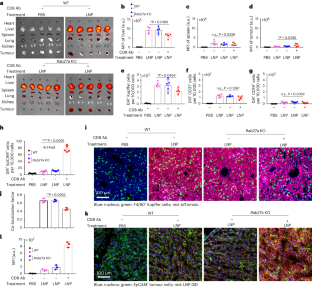
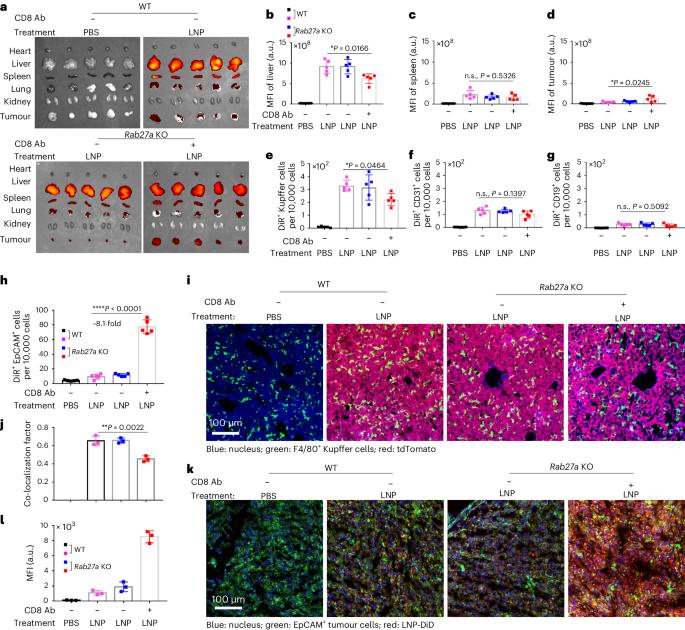
纳米粒子在给药应用方面前景广阔,目前已有几种产品获得临床批准。然而,要在实体瘤中实现纳米颗粒的高积累仍然具有挑战性。在这里,我们展示了肿瘤细胞衍生的小细胞外囊泡(sEVs)阻碍了纳米颗粒向肿瘤的递送,揭示了基于纳米颗粒的肿瘤治疗的另一个障碍。肿瘤细胞会在肿瘤微环境中分泌大量的sEVs,然后与进入肿瘤组织的纳米粒子结合,并将它们输送到肝脏Kupffer细胞中降解。抑制 Rab27a(一种控制 sEV 分泌的基因)可降低 sEV 水平,并改善纳米粒子在肿瘤组织中的积累。在脂质纳米颗粒中与 Rab27a 小干扰 RNA 共同封装后,编码抑癌基因和促炎蛋白的信使 RNA 的疗效大大提高。总之,我们的研究结果表明,肿瘤细胞衍生的 sEVs 可作为纳米颗粒肿瘤递送的防御系统,该系统可能是改进基于纳米颗粒的肿瘤疗法的潜在靶点。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Tumour-derived small extracellular vesicles act as a barrier to therapeutic nanoparticle delivery
Nanoparticles are promising for drug delivery applications, with several clinically approved products. However, attaining high nanoparticle accumulation in solid tumours remains challenging. Here we show that tumour cell-derived small extracellular vesicles (sEVs) block nanoparticle delivery to tumours, unveiling another barrier to nanoparticle-based tumour therapy. Tumour cells secrete large amounts of sEVs in the tumour microenvironment, which then bind to nanoparticles entering tumour tissue and traffic them to liver Kupffer cells for degradation. Knockdown of Rab27a, a gene that controls sEV secretion, decreases sEV levels and improves nanoparticle accumulation in tumour tissue. The therapeutic efficacy of messenger RNAs encoding tumour suppressing and proinflammatory proteins is greatly improved when co-encapsulated with Rab27a small interfering RNA in lipid nanoparticles. Together, our results demonstrate that tumour cell-derived sEVs act as a defence system against nanoparticle tumour delivery and that this system may be a potential target for improving nanoparticle-based tumour therapies. Cancer cell-derived small extracellular vesicles bind to therapeutic nanoparticles leading them from tumours to the liver for degradation. This mechanism is another barrier for the development of efficient nanoparticle-based cancer therapies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


