一种口服葡萄糖反应性聚合物复合物,用于在小鼠和猪体内高效安全地输送胰岛素
IF 34.9
1区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
摘要
与目前的胰岛素制剂相反,内源性胰岛素可直接进入门静脉,调节肝脏中的葡萄糖代谢,同时将低血糖症降至最低。在此,我们报告了一种两亲性二嵌段共聚物的合成过程,该共聚物由葡萄糖响应正电段和聚羧基甜菜碱组成。这种聚合物与胰岛素混合后可形成蠕虫状胶束,从而实现胃肠道的高效吸收,并在肝脏中形成葡萄糖反应库。在高血糖条件下,这种聚合物会引发胰岛素的快速释放,从而建立起从门静脉到外周的胰岛素梯度--类似于内源性胰岛素,从而安全地调节血糖。这种胰岛素制剂在链脲佐菌素诱导的 1 型糖尿病小鼠模型中显示出剂量依赖性血糖调节效果,并能在非肥胖糖尿病小鼠中将血糖控制在正常血糖水平一天。此外,该制剂在 1 型糖尿病猪模型中显示出一天的降血糖效果,且无明显低血糖症状,显示出安全有效地治疗 1 型糖尿病的前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
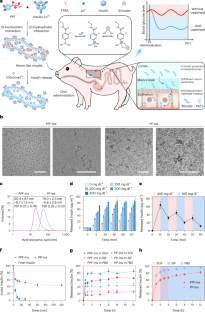

An orally administered glucose-responsive polymeric complex for high-efficiency and safe delivery of insulin in mice and pigs
Contrary to current insulin formulations, endogenous insulin has direct access to the portal vein, regulating glucose metabolism in the liver with minimal hypoglycaemia. Here we report the synthesis of an amphiphilic diblock copolymer comprising a glucose-responsive positively charged segment and polycarboxybetaine. The mixing of this polymer with insulin facilitates the formation of worm-like micelles, achieving highly efficient absorption by the gastrointestinal tract and the creation of a glucose-responsive reservoir in the liver. Under hyperglycaemic conditions, the polymer triggers a rapid release of insulin, establishing a portal-to-peripheral insulin gradient—similarly to endogenous insulin—for the safe regulation of blood glucose. This insulin formulation exhibits a dose-dependent blood-glucose-regulating effect in a streptozotocin-induced mouse model of type 1 diabetes and controls the blood glucose at normoglycaemia for one day in non-obese diabetic mice. In addition, the formulation demonstrates a blood-glucose-lowering effect for one day in a pig model of type 1 diabetes without observable hypoglycaemia, showing promise for the safe and effective management of type 1 diabetes. Orally administrable and glucose-responsive worm-like micelles have been developed to protect insulin in the gastrointestinal tract, enhance its intestinal absorption, accumulate in the liver and enable efficient and safe blood-glucose management.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature nanotechnology
工程技术-材料科学:综合
CiteScore
59.70
自引率
0.80%
发文量
196
审稿时长
4-8 weeks
期刊介绍:
Nature Nanotechnology is a prestigious journal that publishes high-quality papers in various areas of nanoscience and nanotechnology. The journal focuses on the design, characterization, and production of structures, devices, and systems that manipulate and control materials at atomic, molecular, and macromolecular scales. It encompasses both bottom-up and top-down approaches, as well as their combinations.
Furthermore, Nature Nanotechnology fosters the exchange of ideas among researchers from diverse disciplines such as chemistry, physics, material science, biomedical research, engineering, and more. It promotes collaboration at the forefront of this multidisciplinary field. The journal covers a wide range of topics, from fundamental research in physics, chemistry, and biology, including computational work and simulations, to the development of innovative devices and technologies for various industrial sectors such as information technology, medicine, manufacturing, high-performance materials, energy, and environmental technologies. It includes coverage of organic, inorganic, and hybrid materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


