响应式纳米组装的空间封闭光声效应可促进溶酶体膜渗透和三阴性乳腺癌的免疫治疗
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
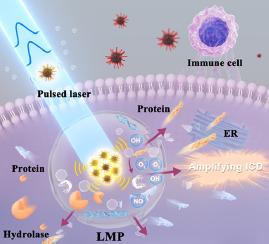
尽管溶酶体膜通透性(LMP)诱导的免疫原性细胞死亡(ICD)能明显提高免疫原性较差的三阴性乳腺癌(TNBC)的抗肿瘤免疫效果,但由于其他死亡途径的发展以及LMP诱导过程中内质网(ER)对溶酶体的修复,其潜力正日益受到限制。本文制备了一种修饰了i-motif DNA并负载了BNN6的聚多巴胺纳米复合材料,通过空间约束光声(PA)效应和一氧化氮的协同作用,促进LMP和TNBC的免疫治疗。将高频脉冲激光(4000 kHz)与纳米复合材料在溶酶体内的组装相结合,产生了空间致密且显著增强的光声效应(比单独分散在细胞外的颗粒高出4.8倍),抑制了对其他细胞成分的损伤,并选择性地将溶酶体完整性降低至19.2%。同时,一氧化氮的释放抑制了ER应激对溶酶体的修复,导致LMP加剧。因此,在体内实现了高效的免疫激活,包括大量释放 CRT/HMGB1(5.93-6.8 倍)、提高树突状细胞的成熟度(3.41 倍)以及促进 CD4+/CD8+ T 细胞的招募(3.99-3.78 倍)。这项研究为合理设计和协同利用封闭能量转换和响应性纳米结构治疗低免疫原性肿瘤开辟了一条新途径。意义说明:为应对三阴性乳腺癌的免疫疗法挑战,我们开发了一种提高溶酶体膜通透性(LMP)并同时防止修复的策略。通过 DNA 引导的溶酶体中多多巴胺纳米复合材料的 pH 响应组装以及高频脉冲激光的应用,实现了空间限制和显著增强的光声(PA)效应。通过分散/组装颗粒的 PA 强度与一氧化氮释放诱导的内质网应激的显著对比,对溶酶体膜造成了选择性的强力破坏,从而保证了高效的免疫性细胞死亡。这项研究为合理设计和协同利用封闭能量转换和响应性纳米结构治疗低免疫原性肿瘤开辟了一条新途径。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spatially confined photoacoustic effects of responsive nanoassembly boosts lysosomal membrane permeabilization and immunotherapy of triple-negative breast cancer
Although immunogenic cell death (ICD) induced by lysosomal membrane permeabilization (LMP) evidently enhance the effectiveness of antitumor immunity for triple-negative breast cancer (TNBC) with poor immunogenicity, their potential is increasingly restricted by the development of other death pathways and the repair of lysosomes by endoplasmic reticulum (ER) during LMP induction. Herein, a polydopamine nanocomposite with i-motif DNA modified and BNN6 loaded is prepared toward boosting LMP and immunotherapy of TNBC by synergy of spatially confined photoacoustic (PA) effects and nitric oxide. Combining the high-frequency pulsed laser (4000 kHz) with the intra-lysosomal assembly of nanocomposites produced spatially confined and significantly boosted PA effects (4.8-fold higher than the individually dispersed particles extracellular), suppressing damage to other cellular components and selectively reducing lysosomal integrity to 19.2 %. Simultaneously, the releasing of nitric oxide inhibited the repair of lysosomes by ER stress, causing exacerbated LMP. Consequently, efficient immune activation was achieved, including the abundant releasing of CRT/HMGB1 (5.93–6.8-fold), the increasing maturation of dendritic cells (3.41-fold), and the fostered recruitment of CD4+/CD8+ T cells (3.99–3.78-fold) in vivo. The study paves a new avenue for the rational design and synergy of confined energy conversion and responsive nanostructures to achieve the treatment of low immunogenicity tumors.
Statement of significance
A strategy of boosting lysosomal membrane permeabilization (LMP) and concomitantly preventing the repair was developed to address the immunotherapy challenge of triple-negative breast cancer. Spatially confined and significantly enhanced photoacoustic (PA) effects were achieved through DNA-guided pH-responsive assembly of polydopamine nanocomposites in lysosomes and application of a high-frequency pulsed laser. Efficient immunogenic cell death was guaranteed by selective and powerful damage of lysosomal membranes through the significant contrast of PA intensities for dispersed/assembled particles and nitric oxide release induced endoplasmic reticulum stress. The study paves a new avenue for the rational design and synergy of confined energy conversion and responsive nanostructures to achieve the treatment of low immunogenicity tumors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


