多反应级联酶催化纳米组件,用于铁变态反应放大和纳米酶辅助温和光热疗法。
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
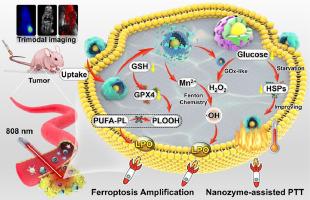
由于活性氧(ROS)生成效率低,铁氧体沉积受到很大限制,而源自热休克蛋白(HSP)的固有自我保护机制严重阻碍了光热疗法(PTT)的效率。在此,我们设计了一种智能策略,利用具有三重酶活性的级联催化纳米组合体(Au@COF@MnO2)放大铁氧化疗法,提高肿瘤光热疗法(PTT)的效率。在共价有机框架(COFs)的帮助下,金纳米酶被封装在中空的二氧化锰(MnO2)外壳中。纳米组合物具有光热转换能力。机理研究表明,Au@COF@MnO2 消耗谷胱甘肽(GSH)会导致谷胱甘肽过氧化物酶 4(GPX4)失活。这种效应与 Mn2+ 介导的活性氧(ROS)生成协同作用,增强了过氧化脂质(LPO)的积累,从而诱导了高效铁变态反应。值得注意的是,纳米金酶促进了葡萄糖向葡萄糖酸和过氧化氢(H2O2)的转化。这一过程提高了芬顿化学反应所需的内源性 H2O2 水平,从而有效促进了 ROS 的生成。同时,葡萄糖耗竭会降低高热诱导的 HSPs 的表达,从而降低细胞的耐热性以增强 PTT。因此,级联催化纳米组件不仅具有很强的肿瘤抑制能力和良好的生物安全性,还具有三模态成像性能,可用于体内成像引导的肿瘤治疗,具有很大的临床应用潜力。意义声明:本研究设计了具有三重酶功能的多响应级联催化纳米组件(Au@COF@MnO2),用于放大铁突变疗法,提高肿瘤 PTT 的效率。该纳米组件具有多响应释放和良好的光热转换性能。葡萄糖消耗诱发的饥饿能下调热疗诱导的 HSPs 在肿瘤细胞中的表达,从而提高 PTT 的疗效。机理研究表明,Au@COF@MnO2对GSH的消耗导致GPX4失活,而GPX4与Mn2+介导的ROS生成协同作用,促进了LPO的积累,从而诱导了高效的铁变态反应。此外,该纳米组件还能在体内进行三模态(PT、PA 和 MR)成像,从而实现纳米组件治疗肿瘤的可视化。这种纳米组件在体内肿瘤治疗中表现出高度的肿瘤抑制性和令人钦佩的生物安全性,具有巨大的临床应用潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Multi-responsive cascade enzyme-like catalytic nanoassembly for ferroptosis amplification and nanozyme-assisted mild photothermal therapy
Ferroptosis is greatly restricted by low reactive oxygen species (ROS) generation efficiency, and the inherent self-protection mechanism originating in heat shock proteins (HSPs) seriously impedes the efficiency of photothermal therapy (PTT). Herein, we designed an intelligent strategy utilizing cascade catalytic nanoassemblies (Au@COF@MnO2) with triple-enzyme activity for amplifying ferroptosis therapy and improving the efficiency of PTT in tumor. Gold nanozyme was encapsulated within a hollow manganese dioxide (MnO2) shell with the help of covalent organic frameworks (COFs). The nanoassemblies possess the ability of photothermal conversion. Mechanism studies suggested that glutathione (GSH) depletion by Au@COF@MnO2 leads to the inactivation of glutathione peroxidase 4 (GPX4). This effect synergized with Mn2+-mediated reactive oxygen species (ROS) generation to enhance the accumulation of lipid peroxide (LPO), thereby inducing high-efficiency ferroptosis. Notably, gold nanozyme facilitated the conversion of glucose into gluconic acid and hydrogen peroxide (H2O2). This process augmented the endogenous H2O2 levels necessary for Fenton chemistry, which could effectively promote the generation of ROS. Simultaneously, glucose depletion downregulated the expression of HSPs induced by hyperthermia, consequently reducing cellular heat resistance for enhancing PTT. Therefore, the cascade catalytic nanoassembly not only exhibits high tumor inhibition and admirable biosafety, but also possesses trimodal imaging performance for imaging-guided tumor therapy in vivo, holding great potential for clinical application.
Statement of significance
This study engineered multi-responsive cascade catalytic nanoassembly (Au@COF@MnO2) with triple enzymatic functions for amplifying ferroptosis therapy and improving the efficiency of PTT in tumor. The nanoassembly exhibited multi-responsive release and great photothermal conversion performance. Glucose consumption-evoked starvation downregulated the hyperthermia-induced expression of HSPs in tumor cells, thereby improving the efficacy of PTT. Mechanism studies suggested that GSH depletion by Au@COF@MnO2 lead to the inactivation of GPX4, which synergized with Mn2+-mediated ROS generation to bolster the accumulation of LPO, thereby inducing high-efficiency ferroptosis. Moreover, the nanoassembly demonstrated trimodal (PT, PA, and MR) imaging in vivo, enabling the visualization of the tumor treatment with nanoassembly. Such nanoassembly exhibited high tumor inhibition and admirable biosafety in tumor therapy in vivo, holding a great potential for clinical application.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


