甲基乙二醛改变胶原纤维的纳米硬度和表面电位
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
胶原纤维是生物组织机械强度和功能的基础。然而,胶原纤维易受非酶糖化的影响而发生变化,形成不可逆转的高级糖化终产物(AGEs)。AGEs 会随着衰老和疾病的发生而累积,并对组织力学和细胞与细胞间的相互作用产生不利影响。AGE 交联一方面与胶原纤维硬度和损伤失调有关,另一方面也与胶原蛋白净表面电荷改变以及细胞识别位点受损有关。虽然之前使用开尔文探针力显微镜(KPFM)进行的研究显示了糖化对胶原纤维表面电位(即净电荷)的影响,但对单个和孤立胶原纤维力学、水合作用和表面电位的综合影响还没有记录。在这里,我们利用原子力显微镜(AFM)纳米压痕法和 KPFM 探索了甲基乙二醛(MGO)处理如何影响单个和分离胶原纤维的力学和表面电位。我们的研究结果表明,MGO 处理会显著增加纳米硬度、改变表面电位并改变胶原纤维水平的水合特性。这些发现强调了 AGEs 对胶原纤维理化特性的关键影响,为病理生理机械和生化改变提供了见解,对衰老过程中和糖尿病患者的细胞机械传导产生了影响。意义说明:胶原纤维易发生糖化,即氨基酸与糖发生不可逆的反应。糖化会影响胶原纤维的机械性能和表面化学性质,从而对生物组织的机械性能和细胞与细胞间的相互作用产生不利影响。目前有关单个胶原纤维糖化的研究很少,主要集中在胶原纤维力学(证据相互矛盾)或表面电位方面。在这里,我们采用了一种结合开尔文探针力(KPFM)和原子力显微镜(AFM)的多模态方法,来研究甲基乙二醛糖化如何在相同的单个和分离胶原纤维上诱导结构、机械和表面电位变化。这种方法有助于了解单个胶原纤维水平上的结构-功能关系。本文章由计算机程序翻译,如有差异,请以英文原文为准。
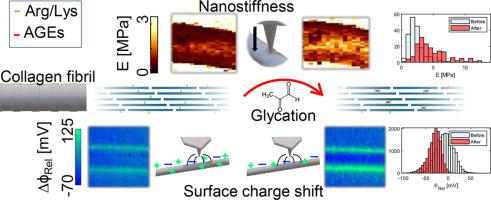
Methylglyoxal alters collagen fibril nanostiffness and surface potential
Collagen fibrils are fundamental to the mechanical strength and function of biological tissues. However, they are susceptible to changes from non-enzymatic glycation, resulting in the formation of advanced glycation end-products (AGEs) that are not reversible. AGEs accumulate with aging and disease and can adversely impact tissue mechanics and cell-ECM interactions. AGE-crosslinks have been related, on the one hand, to dysregulation of collagen fibril stiffness and damage and, on the other hand, to altered collagen net surface charge as well as impaired cell recognition sites. While prior studies using Kelvin probe force microscopy (KPFM) have shown the effect glycation has on collagen fibril surface potential (i.e., net charge), the combined effect on individual and isolated collagen fibril mechanics, hydration, and surface potential has not been documented. Here, we explore how methylglyoxal (MGO) treatment affects the mechanics and surface potential of individual and isolated collagen fibrils by utilizing atomic force microscopy (AFM) nanoindentation and KPFM. Our results reveal that MGO treatment significantly increases nanostiffness, alters surface potential, and modifies hydration characteristics at the collagen fibril level. These findings underscore the critical impact of AGEs on collagen fibril physicochemical properties, offering insights into pathophysiological mechanical and biochemical alterations with implications for cell mechanotransduction during aging and in diabetes.
Statement of significance
Collagen fibrils are susceptible to glycation, the irreversible reaction of amino acids with sugars. Glycation affects the mechanical properties and surface chemistry of collagen fibrils with adverse alterations in biological tissue mechanics and cell-ECM interactions. Current research on glycation, at the level of individual collagen fibrils, is sparse and has focused either on collagen fibril mechanics, with contradicting evidence, or surface potential. Here, we utilized a multimodal approach combining Kelvin probe force (KPFM) and atomic force microscopy (AFM) to examine how methylglyoxal glycation induces structural, mechanical, and surface potential changes on the same individual and isolated collagen fibrils. This approach helps inform structure-function relationships at the level of individual collagen fibrils.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


