产自假孢子菌属一种罕见放线菌的酰化麦芽糖衍生物--孢子苷 A-D。
IF 2.1
4区 医学
Q3 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
摘要
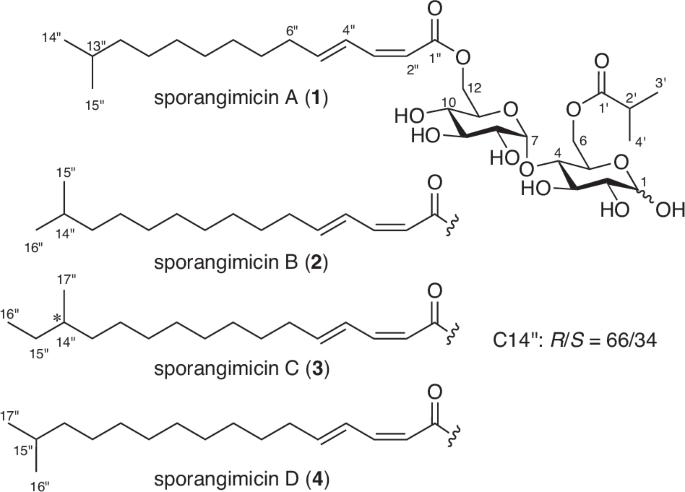
从放线菌 Pseudosporangium sp. RD061809 的培养提取物中发现了四对二酰基二糖的同分异构体--Sporangimicins A-D(1-4),它们代表了一类新的代谢物。化合物 1-4 通过在麦芽糖核心的两个糖 6 位上与一个异丁酰基和一个甲基支链长链二烯酰基的异构体相互转化,导致 HPLC 色谱中的峰分离和核磁共振共振的部分重复。结构阐释的一大亮点是将 Ohrui-Akasaka 方法应用于 3 和 4 在色谱上不可分离的混合物,结果证明 3 和 4 的组成比为 82:18,3 中带有反式异甲基手性中心的 R/S 比为 66:34。化合物 1-4 对革兰氏阳性菌具有抗菌活性,对 P388 小鼠白血病细胞具有适度的细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Sporangimicins A–D, acylated maltose derivatives from a rare actinomycete of the genus Pseudosporangium
Sporangimicins A–D (1–4), four anomeric pairs of diacyl disaccharides that represent a new metabolite class, were discovered from the culture extract of an actinomycete Pseudosporangium sp. RD061809. Compounds 1–4 caused peak separation in the HPLC chromatogram and partial duplication of the NMR resonances by anomeric interconversion of a maltose core modified at the two sugar 6-positions with an isobutanoyl and a methyl-branched long-chain dienoyl groups. A highlight of the structure elucidation was application of Ohrui-Akasaka’s method to a chromatographically inseparable mixture of 3 and 4, which proved the composition ratio of 3 and 4 to be 82:18 and the R/S ratio at the anteiso-methyl bearing chiral center in 3 to be 66:34. Compounds 1–4 showed antimicrobial activity against Gram-positive bacteria and modest cytotoxicity toward P388 murine leukemia cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Antibiotics
医学-免疫学
CiteScore
6.60
自引率
3.00%
发文量
87
审稿时长
1 months
期刊介绍:
The Journal of Antibiotics seeks to promote research on antibiotics and related types of biologically active substances and publishes Articles, Review Articles, Brief Communication, Correspondence and other specially commissioned reports. The Journal of Antibiotics accepts papers on biochemical, chemical, microbiological and pharmacological studies. However, studies regarding human therapy do not fall under the journal’s scope. Contributions regarding recently discovered antibiotics and biologically active microbial products are particularly encouraged. Topics of particular interest within the journal''s scope include, but are not limited to, those listed below:
Discovery of new antibiotics and related types of biologically active substances
Production, isolation, characterization, structural elucidation, chemical synthesis and derivatization, biological activities, mechanisms of action, and structure-activity relationships of antibiotics and related types of biologically active substances
Biosynthesis, bioconversion, taxonomy and genetic studies on producing microorganisms, as well as improvement of production of antibiotics and related types of biologically active substances
Novel physical, chemical, biochemical, microbiological or pharmacological methods for detection, assay, determination, structural elucidation and evaluation of antibiotics and related types of biologically active substances
Newly found properties, mechanisms of action and resistance-development of antibiotics and related types of biologically active substances.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


