Stanniocalcin 1 和 1,25-二羟维生素 D3 可协同调节成骨细胞的骨矿化。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
Stanniocalcin 1(STC1)是一种钙磷调节激素,在包括骨组织在内的所有组织中均有表达,参与钙磷平衡。此前,有研究发现肾近曲小管细胞中 1,25- 二羟维生素 D3 [1,25(OH)2D3] 给药会增加 STC1 的表达。在本研究中,我们探讨了 STC1 是否直接调控成骨细胞分化或相互调控 1,25(OH)2D3 对成骨细胞的影响,从而促进骨稳态。我们发现,STC1可抑制体外成骨细胞分化和体内骨形态发生蛋白2(BMP2)诱导的异位骨形成。此外,1,25(OH)2D3 通过与维生素 D 受体(VDR)的 Stc1 启动子直接结合,增加了 STC1 的表达。STC1 通过抑制成骨细胞中 Akt 磷酸化介导的 VDR 表达上调激活了 1,25(OH)2D3-VDR 信号通路。STC1 进一步增加了 1,25(OH)2D3 对核因子κB 受体激活剂配体(RANKL)分泌的影响,并通过与 1,25(OH)2D3 呈正相关来抑制成骨细胞的分化。在成骨细胞中特异性过表达 STC1 的转基因小鼠的长骨表型与野生型小鼠无显著差异。然而,与野生型小鼠相比,给予 1,25(OH)2D3 会显著降低 STC1 转基因小鼠的骨量。总之,这些结果表明,STC1 对成骨细胞的分化和骨形成具有负向调节作用;然而,STC1 对成骨细胞的抑制作用是短暂的,在正常情况下可以逆转。然而,STC1和1,25(OH)2D3通过1,25(OH)2D3的协同作用可能会抑制成骨细胞的分化,从而降低骨量。本文章由计算机程序翻译,如有差异,请以英文原文为准。
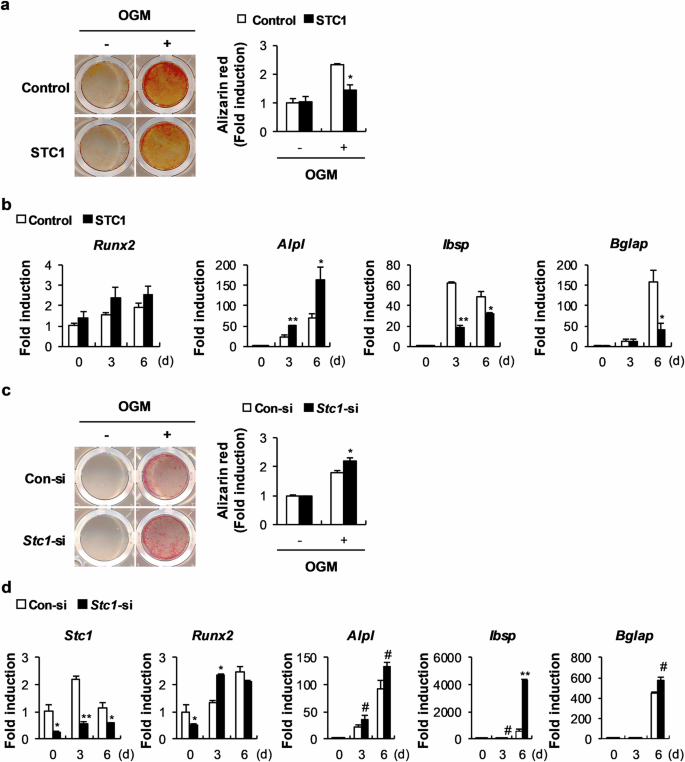
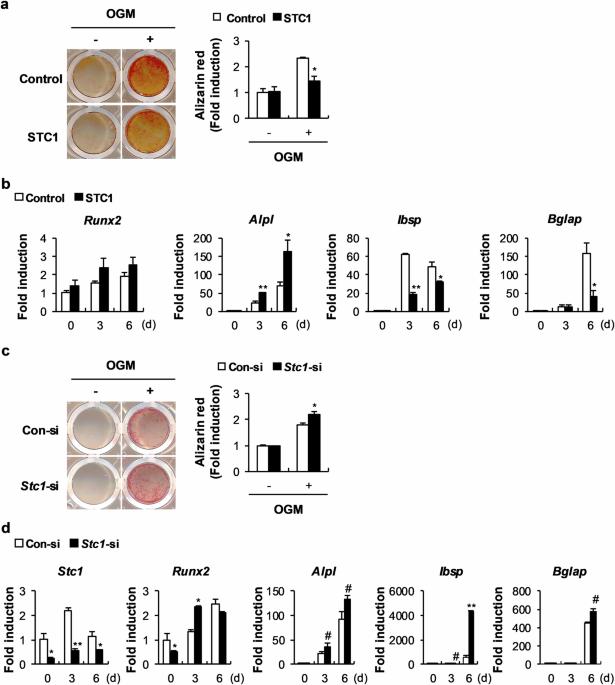
Stanniocalcin 1 and 1,25-dihydroxyvitamin D3 cooperatively regulate bone mineralization by osteoblasts
Stanniocalcin 1 (STC1) is a calcium- and phosphate-regulating hormone that is expressed in all tissues, including bone tissues, and is involved in calcium and phosphate homeostasis. Previously, STC1 expression was found to be increased by 1,25-dihydroxyvitamin D3 [1,25(OH)2D3] administration in renal proximal tubular cells. In this study, we investigated whether STC1 directly regulates osteoblast differentiation or reciprocally controls the effects of 1,25(OH)2D3 on osteoblasts to contribute to bone homeostasis. We found that STC1 inhibited osteoblast differentiation in vitro and bone morphogenetic protein 2 (BMP2)-induced ectopic bone formation in vivo. Moreover, 1,25(OH)2D3 increased STC1 expression through direct binding to the Stc1 promoter of the vitamin D receptor (VDR). STC1 activated the 1,25(OH)2D3–VDR signaling pathway through the upregulation of VDR expression mediated by the inhibition of Akt phosphorylation in osteoblasts. STC1 further increased the effects of 1,25(OH)2D3 on receptor activator of nuclear factor-κB ligand (RANKL) secretion and inhibited osteoblast differentiation by exhibiting a positive correlation with 1,25(OH)2D3. The long-bone phenotype of transgenic mice overexpressing STC1 specifically in osteoblasts was not significantly different from that of wild-type mice. However, compared with that in the wild-type mice, 1,25(OH)2D3 administration significantly decreased bone mass in the STC1 transgenic mice. Collectively, these results suggest that STC1 negatively regulates osteoblast differentiation and bone formation; however, the inhibitory effect of STC1 on osteoblasts is transient and can be reversed under normal conditions. Nevertheless, the synergistic effect of STC1 and 1,25(OH)2D3 through 1,25(OH)2D3 administration may reduce bone mass by inhibiting osteoblast differentiation. In the field of bone health, it’s important to understand how our bodies control bone creation. This research investigates how Stanniocalcin 1 and 1,25-dihydroxyvitamin D3 work together to affect bone mineralization by osteoblasts. The team used mouse models and cell cultures in their experiment. They found that STC1 alone can slow down osteoblast differentiation and bone creation, but this effect is stronger when 1,25-dihydroxyvitamin D3 is also present. This suggests a complex relationship that could affect bone health. They conclude that the combined regulation by STC1 and 1,25-dihydroxyvitamin D3 is a key factor in bone mineralization, providing new insights into managing bone health. This knowledge could lead to treatments for bone diseases by targeting these pathways. Future research may show how to adjust these interactions for treatment benefits, potentially improving results for those with bone health problems. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


