染色质桥解析过程中的Emerin错定位可驱动前列腺癌细胞在富含胶原蛋白的微环境中的侵袭性。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
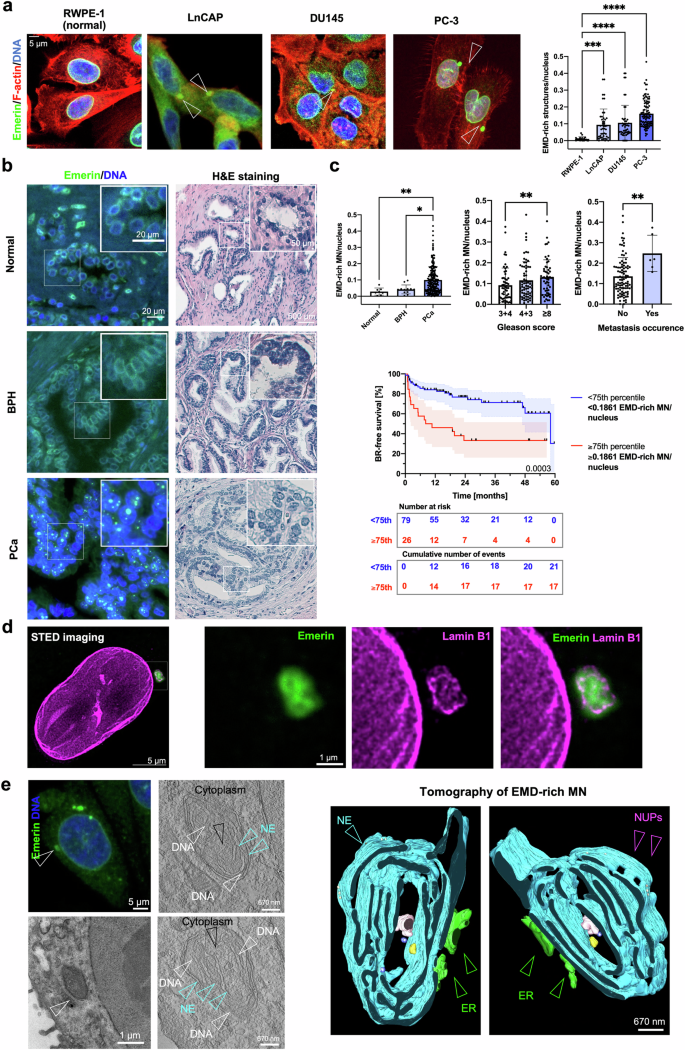
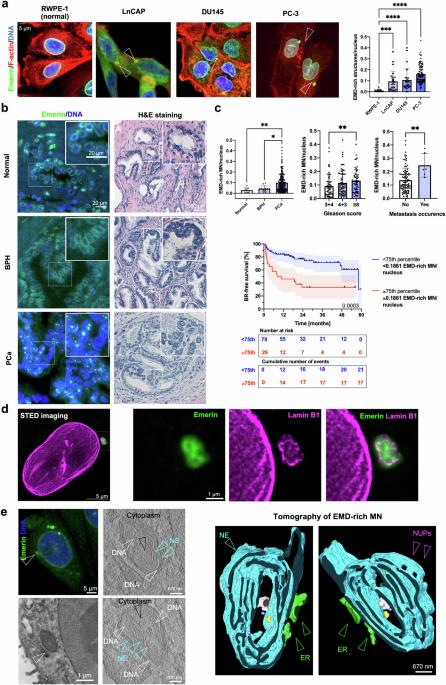
微核(MN)可通过多种机制形成,包括异常细胞染色质桥的断裂。在肿瘤中频繁观察到微核表明,它们可能不仅仅是被动的元素,而是在肿瘤进展中发挥着积极作用。在这里,我们提出了一种机制,通过这种机制,微核的存在可诱导细胞发生特定的表型和功能变化,并增加癌细胞的侵袭潜力。通过整合不同的体外成像和分子技术,并在达米科分类法定义为高风险的前列腺癌(PCa)患者临床样本的支持下,我们证明了染色体桥的解析可导致埃默林的积累和富含埃默林的 MN 的形成。这些结构对层粘蛋白A/C呈阴性,对层粘蛋白B受体和Sec61β呈阳性。MN可作为蛋白质汇,导致核膜上的Emerin贫化。Emerin错定位表型与PCa患者预后不良的分子特征相关,并在转移样本中富集。Emerin错定位与肿瘤细胞迁移和侵袭潜力的增加相对应,尤其是在富含胶原蛋白的微环境中。我们的研究表明,Emerin错定位到MN会导致细胞侵袭性增加,从而恶化患者的预后。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Emerin mislocalization during chromatin bridge resolution can drive prostate cancer cell invasiveness in a collagen-rich microenvironment
Micronuclei (MN) can form through many mechanisms, including the breakage of aberrant cytokinetic chromatin bridges. The frequent observation of MN in tumors suggests that they might not merely be passive elements but could instead play active roles in tumor progression. Here, we propose a mechanism through which the presence of micronuclei could induce specific phenotypic and functional changes in cells and increase the invasive potential of cancer cells. Through the integration of diverse in vitro imaging and molecular techniques supported by clinical samples from patients with prostate cancer (PCa) defined as high-risk by the D’Amico classification, we demonstrate that the resolution of chromosome bridges can result in the accumulation of Emerin and the formation of Emerin-rich MN. These structures are negative for Lamin A/C and positive for the Lamin-B receptor and Sec61β. MN can act as a protein sinks and result in the pauperization of Emerin from the nuclear envelope. The Emerin mislocalization phenotype is associated with a molecular signature that is correlated with a poor prognosis in PCa patients and is enriched in metastatic samples. Emerin mislocalization corresponds with increases in the migratory and invasive potential of tumor cells, especially in a collagen-rich microenvironment. Our study demonstrates that the mislocalization of Emerin to MN results in increased cell invasiveness, thereby worsening patient prognosis. Micronuclei, small nuclei formed during incorrect cell division, can signal genomic instability, often found in cancer. This research investigates how micronuclei may contribute to cancer progression, in the context of prostate cancer, by focusing on the nuclear envelope protein, Emerin. The team conducted experiments using different cell lines and patient samples to understand how Emerin’s misplacement from nuclear envelope to micronuclei can impact cancer cell behavior. The study involved analyzing cells for Emerin distribution, observing the effects of Emerin misplacement on cell movement and invasiveness, and examining prostate cancer tissue to see how these findings relate to the disease in patients. It was observed that the presence of Emerin-rich micronuclei correlates to more aggressive cancer behavior and could predict worse outcomes. Targeting the pathways leading to Emerin misplacement could offer novel strategies for treating aggressive prostate cancer. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


