CMTM6 介导沃伯格效应并促进结直肠癌的肝转移。
IF 9.5
2区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
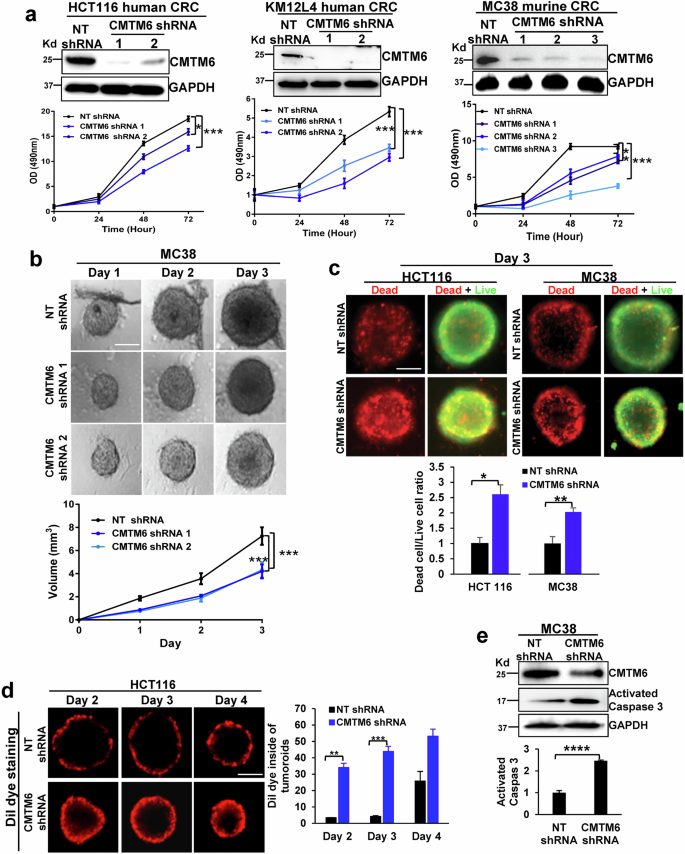
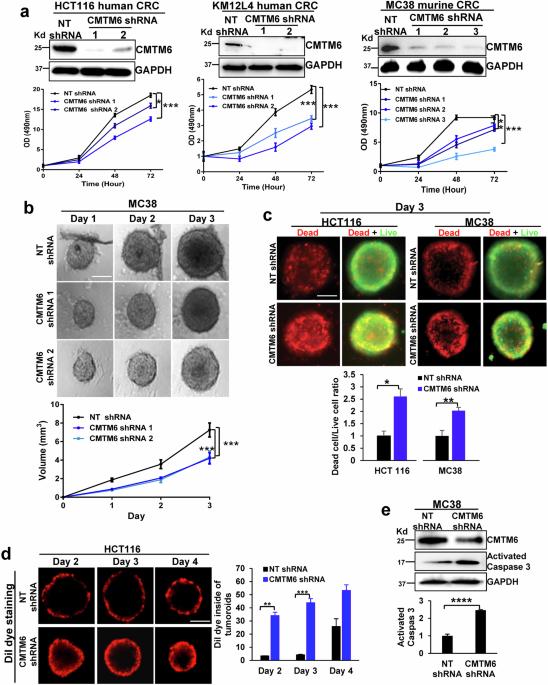
结肠直肠癌(CRC)的肝转移是导致癌症患者死亡的主要原因。在 CRC 肝转移灶中经常观察到葡萄糖转运体 1(Glut1)的过表达和与沃伯格效应相关的葡萄糖摄取增强,但对其潜在机制仍知之甚少。CKLF-like MARVEL跨膜结构域含蛋白6(CMTM6)调控程序性死亡配体-1(PD-L1)的胞内转运;因此,我们研究了CMTM6是否调控CRC细胞中Glut1的转运和沃伯格效应。我们发现,通过 shRNA 敲除 CMTM6 可诱导 Glut1 的溶酶体降解,降低 CRC 细胞的葡萄糖摄取和糖酵解,抑制裸鼠皮下 CRC 生长和 C57BL/6 小鼠的肝转移。从机理上讲,CMTM6与CRC细胞内体中的Glut1和Rab11形成复合物,该复合物是Rab11依赖性转运Glut1至质膜和保护Glut1免于溶酶体降解所必需的。多组学揭示了CMTM6敲除的CRC细胞中的全局转录组变化,这些变化影响了来自CRC肝转移灶的邻近癌相关成纤维细胞的转录组。由于这些转录组变化,CMTM6-敲除的 CRC 细胞表现出 G2 到 M 期转变缺陷、60 种细胞因子/趋化因子分泌减少以及无法招募癌相关成纤维细胞以支持免疫抑制性 CRC 肝转移微环境。对 TCGA 数据的分析证实,CMTM6 在 CRC 患者中的表达增加,而 CMTM6 表达的升高与患者生存率的降低有关。总之,我们的数据表明,CMTM6 在调控 CRC 的沃伯格效应、转录组和肝脏转移方面发挥着多重作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
CMTM6 mediates the Warburg effect and promotes the liver metastasis of colorectal cancer
Liver metastasis of colorectal cancer (CRC) is a leading cause of death among cancer patients. The overexpression of glucose transporter 1 (Glut1) and enhanced glucose uptake that are associated with the Warburg effect are frequently observed in CRC liver metastases, but the underlying mechanisms remain poorly understood. CKLF-like MARVEL transmembrane domain-containing protein 6 (CMTM6) regulates the intracellular trafficking of programmed death-ligand-1 (PD-L1); therefore, we investigated whether CMTM6 regulates Glut1 trafficking and the Warburg effect in CRC cells. We found that knocking down of CMTM6 by shRNA induced the lysosomal degradation of Glut1, decreased glucose uptake and glycolysis in CRC cells, and suppressed subcutaneous CRC growth in nude mice and liver metastasis in C57BL/6 mice. Mechanistically, CMTM6 forms a complex with Glut1 and Rab11 in the endosomes of CRC cells, and this complex is required for the Rab11-dependent transport of Glut1 to the plasma membrane and for the protection of Glut1 from lysosomal degradation. Multiomics revealed global transcriptomic changes in CMTM6-knockdown CRC cells that affected the transcriptomes of adjacent cancer-associated fibroblasts from CRC liver metastases. As a result of these transcriptomic changes, CMTM6-knockdown CRC cells exhibited a defect in the G2-to-M phase transition, reduced secretion of 60 cytokines/chemokines, and inability to recruit cancer-associated fibroblasts to support an immunosuppressive CRC liver metastasis microenvironment. Analysis of TCGA data confirmed that CMTM6 expression was increased in CRC patients and that elevated CMTM6 expression was associated with worse patient survival. Together, our data suggest that CMTM6 plays multiple roles in regulating the Warburg effect, transcriptome, and liver metastasis of CRC. Liver metastasis in colorectal cancer patients increases death rates, with current treatments often inadequate due to a lack of understanding of the underlying processes. This study explores how CRC cells change their metabolism to survive in the liver, focusing on the Warburg effect, where cancer cells use glycolysis preferentially. It focuses on the role of a protein called CMTM6 in this metabolic change. The researchers performed experiments on human and mouse CRC cells and used both in vitro and in vivo models, including mice with and without immune systems, to study the effects of CMTM6 on CRC growth and liver metastasis. The results showed reducing CMTM6 levels led to decreased glycolysis of CRC cells, CRC tumor growth and liver metastasis in mice. Future studies could lead to more effective treatments for CRC patients with liver metastases. This summary was initially drafted using artificial intelligence, then revised and fact-checked by the author.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Experimental and Molecular Medicine
医学-生化与分子生物学
CiteScore
19.50
自引率
0.80%
发文量
166
审稿时长
3 months
期刊介绍:
Experimental & Molecular Medicine (EMM) stands as Korea's pioneering biochemistry journal, established in 1964 and rejuvenated in 1996 as an Open Access, fully peer-reviewed international journal. Dedicated to advancing translational research and showcasing recent breakthroughs in the biomedical realm, EMM invites submissions encompassing genetic, molecular, and cellular studies of human physiology and diseases. Emphasizing the correlation between experimental and translational research and enhanced clinical benefits, the journal actively encourages contributions employing specific molecular tools. Welcoming studies that bridge basic discoveries with clinical relevance, alongside articles demonstrating clear in vivo significance and novelty, Experimental & Molecular Medicine proudly serves as an open-access, online-only repository of cutting-edge medical research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


