髓过氧化物酶-ANCA IgG 会根据协同免疫刺激的类型诱发不同形式的小血管炎。
IF 14.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
抗中性粒细胞胞浆自身抗体(ANCA)血管炎的损伤模式多种多样,包括显微镜下多血管炎(MPA)、肉芽肿伴多血管炎(GPA)和嗜酸粒细胞肉芽肿伴多血管炎(EGPA)。坏死性和新月体性肾小球肾炎(NCGN)发生在所有综合征中,并表现为肾局限性血管炎(RLV)。小鼠髓过氧化物酶(MPO)特异性 ANCA IgG 单剂量静脉注射可引起小鼠 RLV。尽管多种小鼠模型阐明了 ANCA IgG 诱导的坏死性和新月体性肾小球肾炎(NCGN),但 ANCA 诱导的肾外肉芽肿和血管炎的发病机制尚未明确。为了研究这个问题,我们在同一品系的小鼠中静脉注射 MPO-ANCA IgG,诱发了不同模式的肺部疾病,这些疾病与肉芽肿伴多血管炎(GPA)、显微镜下多血管炎(MPA)和嗜酸性肉芽肿伴多血管炎(EGPA)患者的肺部疾病相似。重复静脉注射 MPO-ANCA IgG 可诱导 GPA 伴 NCGN、肺毛细血管炎、动脉炎和肉芽肿病。通过免疫组化图像分析和流式细胞术评估了肺白细胞表型。ANCA 肺毛细血管炎和微脓肿在一天内开始出现,并在七天内演变为肉芽肿。流感加单剂量 MPO-ANCA IgG 可诱发伴有 NCGN、肺毛细血管炎和动脉炎的 MPA,但不伴有肉芽肿。由屋尘螨或卵清蛋白引起的过敏性气道疾病加上单剂量静脉注射 MPO-ANCA IgG 可诱发 EGPA,并伴有嗜酸性粒细胞性支气管炎、NCGN、毛细血管炎、动脉炎和肉芽肿病。因此,我们的研究表明,肺部病变的发生和模式是由相同的 ANCA IgG 和不同的协同免疫因素共同决定的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
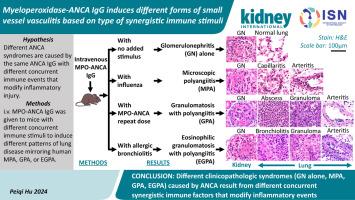
Myeloperoxidase-ANCA IgG induces different forms of small vessel vasculitis based on type of synergistic immune stimuli
Anti-neutrophil cytoplasmic autoantibody (ANCA) vasculitis has diverse patterns of injury including microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Necrotizing and crescentic glomerulonephritis (NCGN) occurs in all syndromes and as renal limited vasculitis (RLV). Single-dose intravenous ANCA IgG-specific for mouse myeloperoxidase (MPO) causes RLV in mice. Although multiple mouse models have elucidated ANCA–IgG induced necrotizing and crescentic glomerulonephritis (NCGN), pathogenesis of ANCA-induced granulomatosis and vasculitis outside the kidney has not been clarified. To investigate this, we used intravenous MPO-ANCA IgG in the same strain of mice to induce different patterns of lung disease mirroring patients with granulomatosis with polyangiitis (GPA), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA). Repeated intravenous MPO-ANCA IgG induced GPA with NCGN, lung capillaritis, arteritis and granulomatosis. Lung leukocyte phenotypes were evaluated by immunohistochemical image analysis and by flow cytometry. ANCA lung capillaritis and microabscesses began within one day and evolved into granulomas in under seven days. Influenza plus single-dose MPO-ANCA IgG induced MPA with NCGN, lung capillaritis and arteritis, but no granulomatosis. Allergic airway disease caused by house dust mites or ovalbumin plus single-dose intravenous MPO-ANCA IgG induced EGPA with eosinophilic bronchiolitis, NCGN, capillaritis, arteritis, and granulomatosis. Thus, our study shows that the occurrence and pattern of lung lesions are determined by the same ANCA IgG accompanied by different synergistic immune factors.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney international
医学-泌尿学与肾脏学
CiteScore
23.30
自引率
3.10%
发文量
490
审稿时长
3-6 weeks
期刊介绍:
Kidney International (KI), the official journal of the International Society of Nephrology, is led by Dr. Pierre Ronco (Paris, France) and stands as one of nephrology's most cited and esteemed publications worldwide.
KI provides exceptional benefits for both readers and authors, featuring highly cited original articles, focused reviews, cutting-edge imaging techniques, and lively discussions on controversial topics.
The journal is dedicated to kidney research, serving researchers, clinical investigators, and practicing nephrologists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


