一项随机、开放标签临床试验采用个体干预前 eGFR 斜率,考察了卡格列净对白蛋白尿和 eGFR 下降的影响。
IF 14.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
要证明药物在延缓肾病进展方面的疗效,需要针对慢性肾病(CKD)早期患者开展大型临床试验。在这项随机、平行组、开放标签试验(CANPIONE 研究)中,我们使用个体在治疗前(干预前斜率)和治疗期间(慢性斜率)的估计肾小球滤过率(eGFR)斜率变化来评估钠-葡萄糖共转运体 2(SGLT2)抑制剂卡格列净的疗效。我们随机分配(1:1)患有2型糖尿病、尿白蛋白与肌酐比值(UACR)在50至300毫克/克以下、eGFR至少为45毫升/分钟/1.73平方米的参与者接受卡格列净或除SGLT2抑制剂以外的指南推荐治疗(对照组)。第一项和第二项主要结果分别是 UACR 与基线相比的几何平均百分比变化和 eGFR 斜率变化。在 98 名随机参与者中,96 人接受了至少一种研究治疗。卡格列净组的 UACR 对数变换几何平均值与基线相比的最小二乘法平均值变化显著大于对照组(组间差异为-30.8%(95% 置信区间为-42.6 至-16.8)。eGFR斜率变化(慢性期-干预前)的组间差异(卡格列非洛嗪组-对照组)为每年4.4(1.6-7.3)毫升/分钟/1.73平方米,这在eGFR下降较快的参与者中更为明显。总之,卡格列净降低了白蛋白尿,并减少了2型糖尿病和微量白蛋白尿患者eGFR下降的特异性自然过程。因此,CANPIONE 研究表明,eGFR 斜率的个体内变化可能是确定新疗法在 CKD 早期阶段保护肾脏潜力的一种新方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
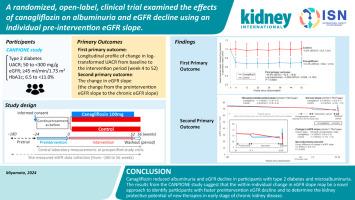
A randomized, open-label, clinical trial examined the effects of canagliflozin on albuminuria and eGFR decline using an individual pre-intervention eGFR slope
Demonstrating drug efficacy in slowing kidney disease progression requires large clinical trials when targeting participants with an early stage of chronic kidney disease (CKD). In this randomized, parallel-group, open-labeled trial (CANPIONE study), we assessed the effect of the sodium-glucose cotransporter 2 (SGLT2) inhibitor canagliflozin using the individual’s change in estimated glomerular filtration rate (eGFR) slope before (pre-intervention slope) and during treatment (chronic slope). We randomly assigned (1:1) participants with type 2 diabetes, urinary albumin-to-creatinine ratio (UACR) of 50 to under 300 mg/g, and an eGFR of at least 45 ml/min/1.73m2 to receive canagliflozin or guideline-recommended treatment except for SGLT2 inhibitors (control). The first and second primary outcomes were the geometric mean percentage change from baseline in UACR and the change in eGFR slope, respectively. Of 98 randomized participants, 96 received at least one study treatment. The least-squares mean change from baseline in log-transformed geometric mean UACR was significantly greater in the canagliflozin group than the control group (between group-difference, −30.8% (95% confidence interval −42.6 to −16.8). The between-group difference (canagliflozin group – control group) of change in eGFR slope (chronic – pre-intervention) was 4.4 (1.6 to 7.3) ml/min/1.73 m2 per year, which was more pronounced in participants with faster eGFR decline. In summary, canagliflozin reduced albuminuria and the participant-specific natural course of eGFR decline in participants with type 2 diabetes and microalbuminuria. Thus, the CANPIONE study suggests that the within-individual change in eGFR slope may be a novel approach to determine the kidney protective potential of new therapies in early stages of CKD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney international
医学-泌尿学与肾脏学
CiteScore
23.30
自引率
3.10%
发文量
490
审稿时长
3-6 weeks
期刊介绍:
Kidney International (KI), the official journal of the International Society of Nephrology, is led by Dr. Pierre Ronco (Paris, France) and stands as one of nephrology's most cited and esteemed publications worldwide.
KI provides exceptional benefits for both readers and authors, featuring highly cited original articles, focused reviews, cutting-edge imaging techniques, and lively discussions on controversial topics.
The journal is dedicated to kidney research, serving researchers, clinical investigators, and practicing nephrologists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


