一项 2b 期随机试验研究了胰高血糖素样肽-1 和胰高血糖素受体激动剂可他鲁肽对糖尿病肾病患者肾脏疗效的影响。
IF 14.8
1区 医学
Q1 UROLOGY & NEPHROLOGY
引用次数: 0
摘要
科他杜肽是一种胰高血糖素样肽-1(GLP-1)和胰高血糖素受体激动剂,可改善2型糖尿病(T2D)和慢性肾病(CKD)患者的肾功能。在这项 2b 期研究中,T2D 和 CKD 患者(估计肾小球滤过率 [eGFR] 为 20 或以上且低于 90 毫升/分钟/1.共同主要终点是 UACR 与安慰剂相比从基线到第 14 周末的绝对值和百分比变化。在248名随机患者中,平均年龄为67.1岁,19%为女性,平均eGFR为55.3 mL/min per 1.73 m2,几何平均UACR为205.5 mg/g(变异系数为270.0),46.8%的患者同时服用钠-葡萄糖协同转运体2抑制剂。与安慰剂相比,从基线到第14周末,科他杜肽剂量依赖性地降低了UACR,在300 μg(-43.9%[95%置信区间-54.7至-30.6])和600 μg(-49.9%[-59.3至-38.4])时达到显著水平;效果持续到第26周。各组的严重不良事件发生率均衡。科他鲁肽 600 μg 的安全性和耐受性与塞马鲁肽相当。因此,我们的研究表明,对于患有 T2D 和慢性肾脏病的患者,在标准治疗的基础上,科他杜肽能显著降低 UACR,而且耐受性良好,这表明该药具有保护肾脏的功效,需要在更大规模的研究中加以证实。本文章由计算机程序翻译,如有差异,请以英文原文为准。
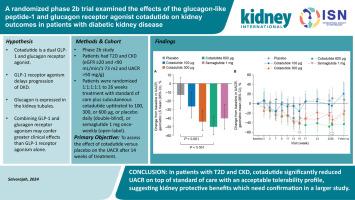
A randomized phase 2b trial examined the effects of the glucagon-like peptide-1 and glucagon receptor agonist cotadutide on kidney outcomes in patients with diabetic kidney disease
Cotadutide is a glucagon-like peptide-1 (GLP-1) and glucagon receptor agonist that may improve kidney function in patients with type 2 diabetes (T2D) and chronic kidney disease (CKD). In this phase 2b study, patients with T2D and CKD (estimated glomerular filtration rate [eGFR] of 20 or more and under 90 mL/min per 1.73 m2 and urinary albumin-to-creatinine ratio [UACR] over 50 mg/g) were randomized 1:1:1:1:1 to 26 weeks’ treatment with standard of care plus subcutaneous cotadutide uptitrated to 100, 300, or 600 μg, or placebo daily (double-blind), or the GLP-1 agonist semaglutide 1 mg once weekly (open-label).The co-primary endpoints were absolute and percentage change versus placebo in UACR from baseline to the end of week 14. Among 248 randomized patients, mean age 67.1 years, 19% were female, mean eGFR was 55.3 mL/min per 1.73 m2, geometric mean was UACR 205.5 mg/g (coefficient of variation 270.0), and 46.8% were receiving concomitant sodium–glucose co-transporter 2 inhibitors. Cotadutide dose-dependently reduced UACR from baseline to the end of week 14, reaching significance at 300 μg (–43.9% [95% confidence interval −54.7 to −30.6]) and 600 μg (−49.9% [−59.3 to −38.4]) versus placebo; with effects sustained at week 26. Serious adverse events were balanced across arms. Safety and tolerability of cotadutide 600 μg were comparable to semaglutide. Thus, our study shows that in patients with T2D and CKD, cotadutide significantly reduced UACR on top of standard of care with an acceptable tolerability profile, suggesting kidney protective benefits that need confirmation in a larger study.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Kidney international
医学-泌尿学与肾脏学
CiteScore
23.30
自引率
3.10%
发文量
490
审稿时长
3-6 weeks
期刊介绍:
Kidney International (KI), the official journal of the International Society of Nephrology, is led by Dr. Pierre Ronco (Paris, France) and stands as one of nephrology's most cited and esteemed publications worldwide.
KI provides exceptional benefits for both readers and authors, featuring highly cited original articles, focused reviews, cutting-edge imaging techniques, and lively discussions on controversial topics.
The journal is dedicated to kidney research, serving researchers, clinical investigators, and practicing nephrologists.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


