整合机器学习生存框架,破解用于预测肺腺癌预后的多种细胞死亡模式。
IF 4.5
3区 医学
Q1 GENETICS & HEREDITY
引用次数: 0
摘要
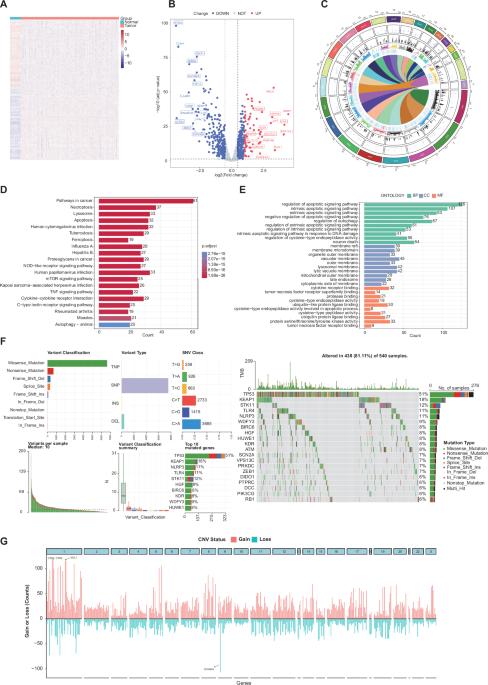
各种形式的程序性细胞死亡(PCD)共同调控着肿瘤的发生、发展和转移。然而,目前还缺乏对肺腺癌(LUAD)中各种类型的程序性细胞死亡的全面分析。这项研究涵盖了与 13 种不同 PCD 模式调控相关的 1481 个基因。研究人员将十种机器学习算法合并成101种组合,从中选出最优算法,根据四个多中心队列的平均C指数制定人工智能衍生预后特征。已建立的最佳细胞死亡指数(CDI)模型成为总生存期的独立风险因素,表现出稳健而一致的性能。值得注意的是,与传统的临床变量和分子特征相比,CDI的准确性明显更高。与其他已发表的模型相比,它表现出更优越的性能。通过将 CDI 与相关临床特征相结合,我们开发出了一个具有出色预测性能的提名图。低 CDI 得分的 LUAD 患者的免疫调节剂、TIDE 评分和免疫评分均较高,表明其免疫治疗效果更好。更重要的是,我们发现抗原呈递调控是 PCD 的关键机制。SCG2是抑制LUAD恶性进展的关键分子。CDI作为一种强大而有前途的工具,在提高LUAD患者的临床疗效方面具有巨大潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Integrated machine learning survival framework to decipher diverse cell death patterns for predicting prognosis in lung adenocarcinoma
Various forms of programmed cell death (PCD) collectively regulate the occurrence, development and metastasis of tumors. Nevertheless, a comprehensive analysis of the diverse types of PCD in lung adenocarcinoma (LUAD) is currently lacking. The study encompassed a total of 1481 genes associated with the regulation of 13 distinct PCD patterns. Ten machine learning algorithms were amalgamated into 101 combinations, from which the optimal algorithm was chosen to formulate an artificial intelligence-derived prognostic signature based on the average C-index across four multicenter cohorts. The established optimal cell death index (CDI) model emerged as an independent risk factor for overall survival, demonstrating robust and consistent performance. Notably, CDI exhibited significantly higher accuracy compared to traditional clinical variables and molecular features. It exhibited superior performance than other published models. By integrating CDI with relevant clinical features, a nomogram with excellent predictive performance was developed. LUAD patients with low CDI score had a higher immune modulators, TIDE scores and immune scores, indicating a better immunotherapy benefit. More importantly, we found that the regulation of antigen presentation is the crucial mechanism of PCD. SCG2 is a key molecule that inhibits the malignant progression of LUAD. CDI holds great potential as a robust and promising tool for enhancing clinical outcomes in patients with LUAD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Genes and immunity
医学-免疫学
CiteScore
8.90
自引率
4.00%
发文量
28
审稿时长
6-12 weeks
期刊介绍:
Genes & Immunity emphasizes studies investigating how genetic, genomic and functional variations affect immune cells and the immune system, and associated processes in the regulation of health and disease. It further highlights articles on the transcriptional and posttranslational control of gene products involved in signaling pathways regulating immune cells, and protective and destructive immune responses.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


