酸促进的自光催化区域选择性氧化:获取喹喔啉-2,3-二酮的新策略。
IF 3.7
2区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文公开了一种利用空气(O2)作为绿色氧化剂,通过酸促进喹喔啉-2(1H)-酮在 C-3 位的自光催化区域选择性氧化制备喹喔啉-2,3-二酮的有效方法。该方案为制备喹喔啉-2,3-二酮衍生物提供了一条新的合成路线,其特点是反应条件温和、操作简单、底物范围广,无需外加光催化剂、金属试剂和强氧化剂。本文章由计算机程序翻译,如有差异,请以英文原文为准。

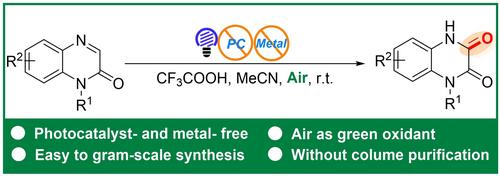
Acid-Promoted Self-Photocatalyzed Regioselective Oxidation: A Novel Strategy for Accessing Quinoxaline-2,3-diones
Disclosed here is an efficient approach for the preparation of quinoxaline-2,3-diones using air (O2) as a green oxidant via acid-promoted self-photocatalyzed regioselective oxidation of quinoxalin-2(1H)-ones at C-3 position. This protocol presents a novel synthetic route for the preparation of quinoxaline-2,3-dione derivatives, featuring mild reaction conditions, simple operation, and a wide range of substrates, without the need for external photocatalysts, metal reagents, and strong oxidants.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemistry - A European Journal
化学-化学综合
CiteScore
7.90
自引率
4.70%
发文量
1808
审稿时长
1.8 months
期刊介绍:
Chemistry—A European Journal is a truly international journal with top quality contributions (2018 ISI Impact Factor: 5.16). It publishes a wide range of outstanding Reviews, Minireviews, Concepts, Full Papers, and Communications from all areas of chemistry and related fields.
Based in Europe Chemistry—A European Journal provides an excellent platform for increasing the visibility of European chemistry as well as for featuring the best research from authors from around the world.
All manuscripts are peer-reviewed, and electronic processing ensures accurate reproduction of text and data, plus short publication times.
The Concepts section provides nonspecialist readers with a useful conceptual guide to unfamiliar areas and experts with new angles on familiar problems.
Chemistry—A European Journal is published on behalf of ChemPubSoc Europe, a group of 16 national chemical societies from within Europe, and supported by the Asian Chemical Editorial Societies. The ChemPubSoc Europe family comprises: Angewandte Chemie, Chemistry—A European Journal, European Journal of Organic Chemistry, European Journal of Inorganic Chemistry, ChemPhysChem, ChemBioChem, ChemMedChem, ChemCatChem, ChemSusChem, ChemPlusChem, ChemElectroChem, and ChemistryOpen.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


