口服微纳米基因组编辑系统实现了 CRISPR-Cas9 的靶向传递和条件激活,用于炎症性肠病的基因治疗
IF 15.8
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
强大的CRISPR-Cas9技术可以纠正人类突变细胞中的基因,实现多种疾病的治疗,但它缺乏安全有效的给药系统。在此,我们提出了一种口服微纳米基因组编辑系统,针对肠道中过量的 TNF-α,用于炎症性肠病(IBD)的特异性基因治疗。该编辑系统通过二硫键桥接,将 Cas9/sgRNA 核糖核蛋白(RNP)组装成纳米簇(NC)。随后,RNP-NCs被封装在来自大肠杆菌Nissle 1917的炎症细胞靶向脂多糖去脂外膜囊泡(dOMVs)中,外层的海藻酸钙微球(CAMs)进一步保护了这些囊泡。通过利用 CAMs 的保护作用,口服给药系统在进入胃部后可抵御胃酸降解,从而高效地实现向肠道的靶向给药。随着pH值逐渐升高,微尺度CAMs膨胀并解体,将包裹在dOMVs中的纳米级RNP-NCs释放到肠道中。这些RNP-NCs@dOMVs可穿过粘膜屏障,靶向炎性巨噬细胞,条件激活的Cas9/sgRNA RNPs可有效地在细胞核内对TNF-α进行基因组编辑。这种口服微纳米基因组编辑系统是治疗 IBD 的一个前景广阔的转化平台。本文章由计算机程序翻译,如有差异,请以英文原文为准。
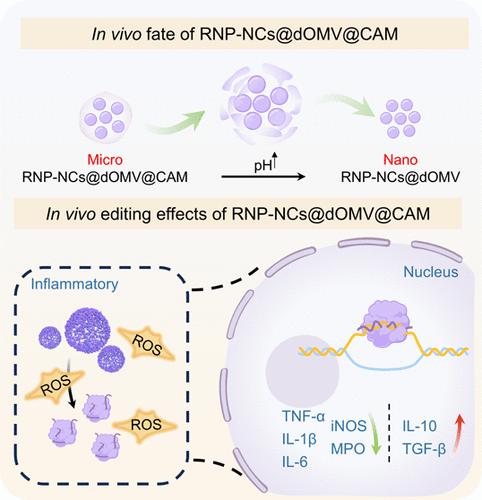
Oral Microto-Nano Genome-Editing System Enabling Targeted Delivery and Conditional Activation of CRISPR-Cas9 for Gene Therapy of Inflammatory Bowel Disease
The potent CRISPR-Cas9 technology can correct genes in human mutated cells to achieve the treatment of multiple diseases, but it lacks safe and effective delivery systems. Herein, we proposed an oral microto-nano genome-editing system aiming at the enteric excessive level of TNF-α for specific gene therapy of inflammatory bowel disease (IBD). This editing system facilitated the assembly of Cas9/sgRNA ribonucleoprotein (RNP) into nanoclusters (NCs) through the bridging of disulfide bonds. RNP-NCs were subsequently encapsulated within inflammatory cell-targeted lipopolysaccharide-deleted outer membrane vesicles (dOMVs) sourced from Escherichia coli Nissle 1917, which were further shielded by an outer layer of calcium alginate microspheres (CAMs). By leveraging the protection effect of CAMs, the oral administration system withstood gastric acid degradation upon entry into the stomach, achieving targeted delivery to the intestines with high efficiency. As the pH gradually rose, the microscale CAMs swelled and disintegrated, releasing nanoscale RNP-NCs encapsulated in dOMVs into the intestines. These RNP-NCs@dOMVs could traverse the mucosal barrier and target inflammatory macrophages where conditionally activated Cas9/sgRNA RNPs effectively perform genomic editing of TNF-α within the nucleus. Such oral microto-nano genome-editing systems represent a promising translational platform for the treatment of IBD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

ACS Nano
工程技术-材料科学:综合
CiteScore
26.00
自引率
4.10%
发文量
1627
审稿时长
1.7 months
期刊介绍:
ACS Nano, published monthly, serves as an international forum for comprehensive articles on nanoscience and nanotechnology research at the intersections of chemistry, biology, materials science, physics, and engineering. The journal fosters communication among scientists in these communities, facilitating collaboration, new research opportunities, and advancements through discoveries. ACS Nano covers synthesis, assembly, characterization, theory, and simulation of nanostructures, nanobiotechnology, nanofabrication, methods and tools for nanoscience and nanotechnology, and self- and directed-assembly. Alongside original research articles, it offers thorough reviews, perspectives on cutting-edge research, and discussions envisioning the future of nanoscience and nanotechnology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


