活细胞中的 µMap 近距离标记揭示了应力颗粒的解体机制
IF 12.9
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
相分离凝聚体是一种无膜细胞内结构,由动态蛋白质相互作用组成,可组织重要的生物过程。了解这些细胞器的组成和动态有助于我们了解与颗粒失调有关的细胞行为和疾病病理。在本研究中,我们利用基于 HaloTag 的平台(HaloMap)绘制微环境图谱,以描述活细胞中细胞内应激颗粒的动态特征。在验证了这种方法的稳健性和灵敏度后,我们对应激颗粒蛋白质组的形成、解体和药理扰动过程进行了剖析。这些实验揭示了几种泛素相关的调节剂,包括 HECT(与 E6AP C 末端同源)E3 连接酶 ITCH 和 NEDD4L,以及泛素受体 toll-interacting 蛋白 TOLLIP,它们是颗粒解体的关键介质。此外,我们还发现了促进颗粒清除的自噬相关途径。总之,这项研究为揭示细胞内蛋白质相互作用组建立了一种通用的光接近标记方法,并揭示了以前未曾探索过的应激颗粒动力学调控机制。本文章由计算机程序翻译,如有差异,请以英文原文为准。

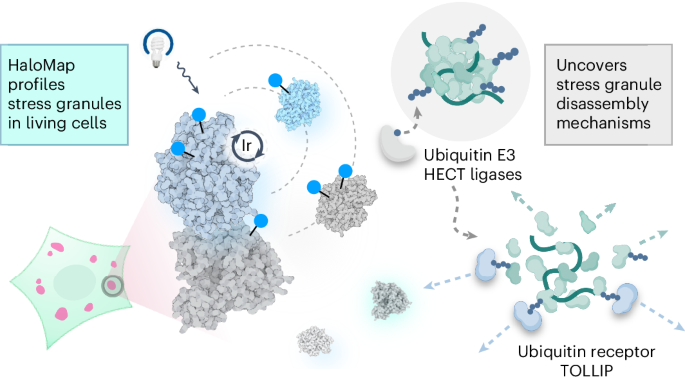
µMap proximity labeling in living cells reveals stress granule disassembly mechanisms
Phase-separated condensates are membrane-less intracellular structures comprising dynamic protein interactions that organize essential biological processes. Understanding the composition and dynamics of these organelles advances our knowledge of cellular behaviors and disease pathologies related to granule dysregulation. In this study, we apply microenvironment mapping with a HaloTag-based platform (HaloMap) to characterize intracellular stress granule dynamics in living cells. After validating the robustness and sensitivity of this approach, we then profile the stress granule proteome throughout the formation and disassembly and under pharmacological perturbation. These experiments reveal several ubiquitin-related modulators, including the HECT (homologous to E6AP C terminus) E3 ligases ITCH and NEDD4L, as well as the ubiquitin receptor toll-interacting protein TOLLIP, as key mediators of granule disassembly. In addition, we identify an autophagy-related pathway that promotes granule clearance. Collectively, this work establishes a general photoproximity labeling approach for unraveling intracellular protein interactomes and uncovers previously unexplored regulatory mechanisms of stress granule dynamics. Pan et al. establish a general photoproximity labeling approach in living cells, applying microenvironment mapping with a HaloTag-based platform (HaloMap) to profile the stress granule proteome and identify ubiquitin-related modulators as key mediators of granule disassembly.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Nature chemical biology
生物-生化与分子生物学
CiteScore
23.90
自引率
1.40%
发文量
238
审稿时长
12 months
期刊介绍:
Nature Chemical Biology stands as an esteemed international monthly journal, offering a prominent platform for the chemical biology community to showcase top-tier original research and commentary. Operating at the crossroads of chemistry, biology, and related disciplines, chemical biology utilizes scientific ideas and approaches to comprehend and manipulate biological systems with molecular precision.
The journal embraces contributions from the growing community of chemical biologists, encompassing insights from chemists applying principles and tools to biological inquiries and biologists striving to comprehend and control molecular-level biological processes. We prioritize studies unveiling significant conceptual or practical advancements in areas where chemistry and biology intersect, emphasizing basic research, especially those reporting novel chemical or biological tools and offering profound molecular-level insights into underlying biological mechanisms.
Nature Chemical Biology also welcomes manuscripts describing applied molecular studies at the chemistry-biology interface due to the broad utility of chemical biology approaches in manipulating or engineering biological systems. Irrespective of scientific focus, we actively seek submissions that creatively blend chemistry and biology, particularly those providing substantial conceptual or methodological breakthroughs with the potential to open innovative research avenues. The journal maintains a robust and impartial review process, emphasizing thorough chemical and biological characterization.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


